22 Nov 2024
“Big and heavy” – TAVI in a severely calcified aortic stenosis with large annulus
This case has been accredited by EBAC with 1 CME credit
An 89-year-old male presented with worsening HF symptoms and a history of permanent AF and chronic renal failure. TTE confirmed severe AS, while coronary angiography revealed single vessel disease. He was referred to a heart valve center for further evaluation, where multislice computed tomography showed a heavily calcified aortic valve and critical anatomical details for treatment planning. How would you treat this patient?

Author
Learning objectives
- To learn how to treat a severely calcified aortic stenosis with large annulus
- To understand how to deal with large annulus using self-expending valve
- To learn how to optimize TAVR expansion by post-dilatation.
Clinical presentation
An 89-year-old male patient presented with progressive heart failure symptoms (NYHA class III-IV) at the emergency department of a referring hospital. Until recently, he had been independently managing his daily life, including housekeeping, lawn mowing and going out for 1-hour walks.
The patient had a history of permanent atrial fibrillation under direct oral anticoagulation as well as hypertension. Moreover, he presented with chronic renal failure (creatinine 1.9 mg/dl, GFR 32 ml/min). Otherwise, there were no relevant comorbidities present.
Clinical examination revealed signs of pulmonary congestion, pleural effusion, mild peripheral edema and explicit systolic murmur suspected for aortic stenosis.
Baseline transthoracic echocardiography
Transthoracic echocardiography confirmed severe aortic stenosis with Vmax 4.5 m/s, mean gradient of 50 mmHg and an effective orifice area of 0.4 cm2, as well as mild concomitant aortic regurgitation. Left ventricular systolic function was found to be mildly reduced (ejection fraction 45-50 %), right ventricular function was preserved and mitral and tricuspid valve showed moderate regurgitation.

Coronary angiography and result after PCI of LAD
Coronary angiography showed single vessel disease with 90 % stenosis in the proximal left anterior descending artery, which was treated with PCI and drug-eluting stent implantation.
The patient was referred to the heart valve center of a tertiary care hospital for further work-up and aortic valve treatment.
Multislice computed tomography (MSCT) work-up
Detailed MSCT measurements are given below.

Hockes puck long axis
Hockes puck short axis

Aortic valve calcium score
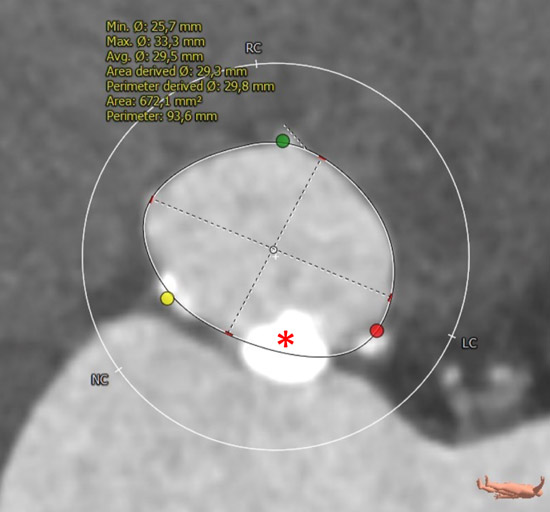
Annular dimensions

LVOT dimensions at 2 mm
LVOT dimensions at 5 mm

Aortic root dimensions
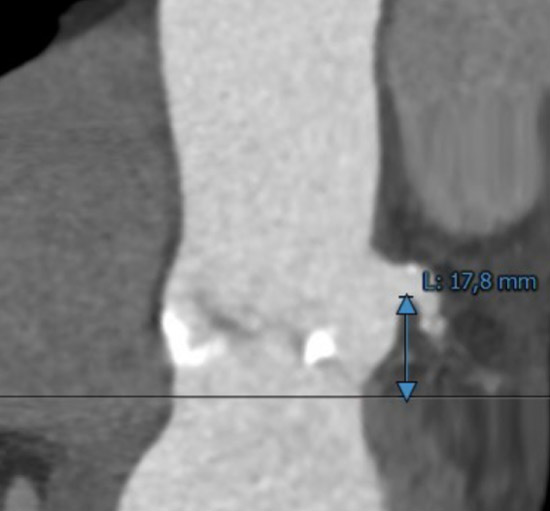
Left coronary ostium height
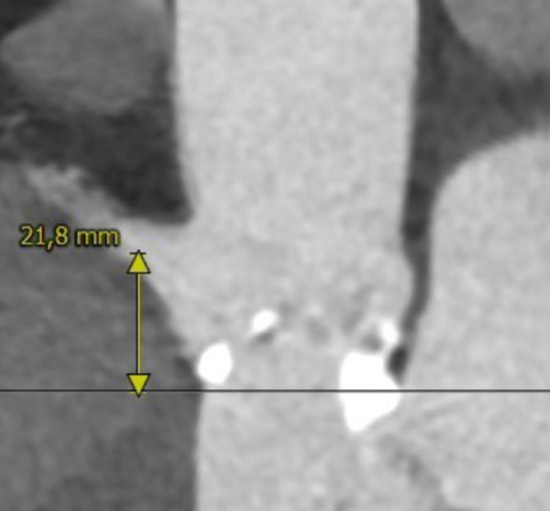
Right coronary ostium height
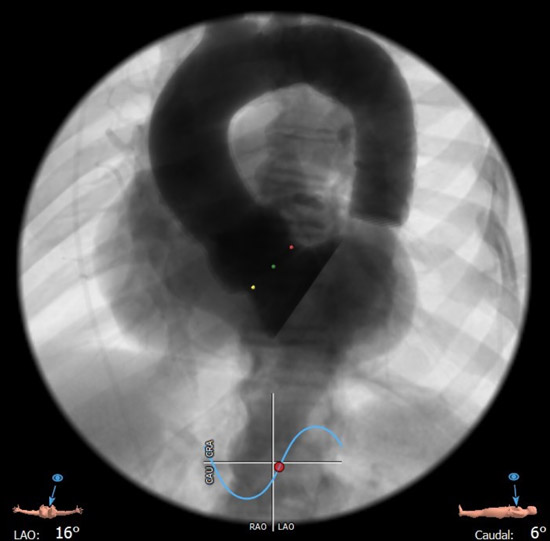
C-arm angulation: 3 cusp view
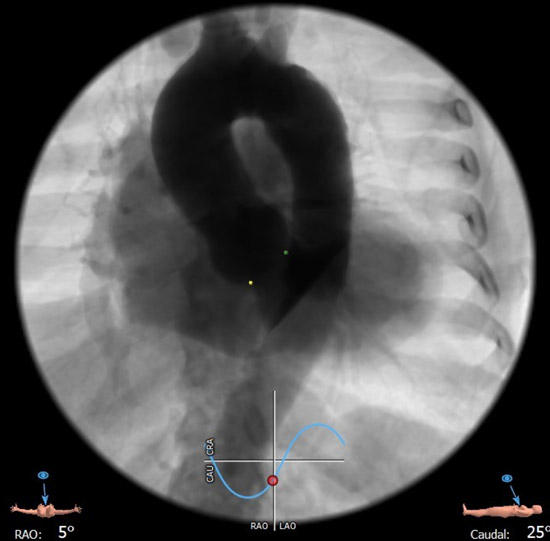
C-arm angulation: Cusp overlap view

Right femoral/iliac access
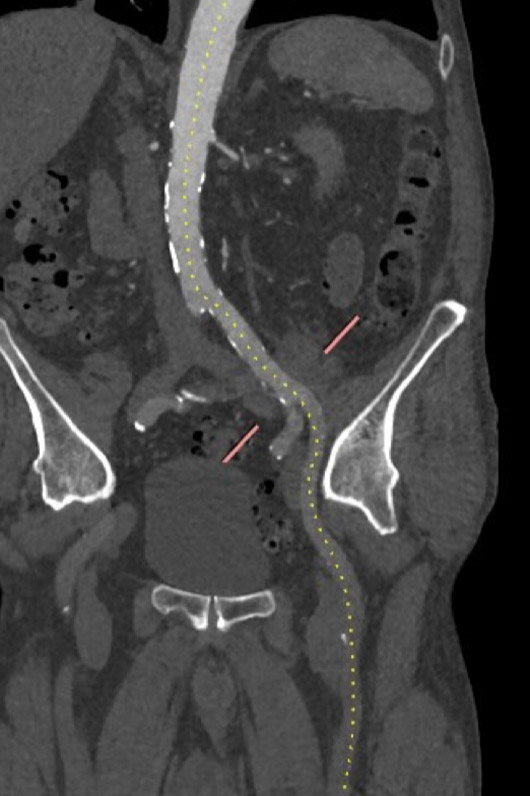
Left femoral/iliac access
MSCT showed a heavily calcified tricuspid aortic valve (aortic valve calcification score: 12,390 A.U.) with a large aortic valve annulus of 29.8 mm perimeter derived diameter (perimeter 93.6 mm, area 672.1 mm2). Moreover, a prominent calcium chunk (marked with asterisk) was present, located at the annular level between the left- and non-coronary cusp and protruding into the LVOT. The ostial heights of the left and right coronary arteries were noncritical (17.8 and 21.8 mm, respectively). Left and right iliac and femoral arteries had a minimal diameter of 7 mm and showed no significant stenosis with moderate tortuosity.
Let us know about your approach!
Heart Team decision
Given the patient’s good physical and mental status, the interdisciplinary Heart Team decided to perform TAVI by using a 35 mm self-expandable intra-annular transcatheter heart valve.
TAVI procedure
TAVI was performed under local anesthesia in the hybrid operating room.
Access for TAVI was obtained by ultrasound-guided puncture of the right common femoral artery, with use of a suture-based vessel closure device (ProStyle™) for pre-closure. A pigtail catheter was placed in the non-coronary sinus via the right radial artery, and a temporary pacing lead was placed in the right ventricle through the right jugular vein.
After crossing the stenotic aortic valve, a pre-shaped stiff wire (Safari S™) was placed in the apex of the left ventricle. Predilatation with a 25 mm Balloon (TrueDilatation™) was performed under rapid pacing to facilitate adequate expansion of the self-expandable transcatheter heart valve within the severely calcified aortic valve. The 35 mm self-expandable intra-annular transcatheter heart valve (Navitor Titan™) was delivered over the aortic arch and implanted under fast pacing by using the cusp overlap technique.
Hemodynamic during valve deployment
During valve deployment, the radiopaque markers were used to achieve the optimal implantation height in relation to the non-coronary (cusp overlap angulation) and the left-coronary (3 cusp angulation) hinge point of the aortic valve.
After confirming adequate valve position, the valve was finally released and the stiff wire was exchanged by a pigtail catheter. No hemodynamic compromise occurred during the implantation process.
Root angiography and fluoroscopy showed residual paravalvular leakage and incomplete expansion of the valve stent frame. Thus, a 26 mm Balloon (TrueDilatation™) was used for post-dilatation, which resulted in full expansion of the stent frame and reduced the amount of aortic regurgitation to trace.
The right femoral access site was closed in the MultiCLOSE technique, by using the pre-inserted ProStyle™ in conjunction with a plaque-based closure device (6F AngioSeal™) with adequate hemostatisis.
Outcome
After in-hospital monitoring for 3 additional days without any signs of high-grade conduction disturbances on ECG, or bleeding complications, the patient was discharged in good clinical condition without residual heart failure symptoms.
Transthoracic echocardiography and ECG at discharge (3rd post-procedural day)
Transthoracic echocardiography at discharge showed only trivial paravalvular leakage of the implanted aortic valve prosthesis, with a mean gradient of 4 mmHg.

Take-home messages
- Use of TAVI in elderly patients with complex anatomies should be based on individual risk-benefit assessment, under consideration of the patients’ physical and mental health status and prognosis.
- Heavily calcified aortic stenosis with large annuli (up to 30 mm diameter) can be safely treated by using the largest intra-annular self-expandable transcatheter heart valve, without any hemodynamic compromise during the valve implantation process.
- When treating severely calcified aortic stenosis with self-expandable valves, incomplete stent frame expansion after valve deployment should be anticipated, and strategies for post-dilatation with proper balloon sizes should be prepared upfront.
The invited Expert's opinion
The case presented by N. Schofer nicely demonstrates state-of-the-art TAVI in an 89-year old male patient with symptomatic severe aortic stenosis.
Neither his advanced age nor his comorbidities such as atrial fibrillation, chronic renal failure, and slightly reduced LV systolic function nor his clinical status - he is still independently managing his daily life – would in my opinion justify conservative treatment, or pure palliative aortic balloon angioplasty.
Accordingly, the patient was transferred to a heart valve center for diagnostic work-up and aortic valve treatment. During the course of the work-up, coronary angiography revealed a 90% stenosis in the proximal LAD, which was immediately treated with DES-PCI. Although some TAVI prostheses might make access to the coronary ostia in future PCI more challenging, there is no evidence from randomized studies that patients benefit from revascularization before TAVI. Instead, PCI in patients with severe aortic stenosis has to be considered (1) a rather high-risk procedure and (2) requires dual or even triple antithrombotic treatment, putting a significantly higher bleeding risk to the patient during the following TAVI procedure.
Detailed analysis of the patient’s CT scan is crucial to successful TAVI. In the presented case a severely calcified tricuspid aortic valve with a large annulus of 29.8 mm perimeter derived diameter and an area of 672.1 mm² was detected with a prominent calcium chunk protruding into the LVOT. To treat this “big and heavy” aortic valve, TAVI was performed with a 35 mm self-expandable intra-annular transcatheter heart valve. I would also have chosen a self-expandable TAVI prosthesis because of the observed calcium chunk protruding into the LVOT, which is thought to put a higher risk of annular rupture during TAVI implantation to the patient, especially if a balloon-expandable valve is used with an overfilled balloon. With the chosen 35 mm prosthesis, aortic annulus diameters up to 30 mm and valve areas up to 707 mm² can be treated, which is well within the range of the presented case.
By watching the case, one can easily learn how to safely and effectively perform TAVI in a severely calcified aortic valve with a very large annulus. (1) Pre-dilation of the aortic valve with a 25 mm balloon was used to facilitate full expansion of the self-expandable prosthesis in the heavily calcified native valve. (2) The “cusp-overlap” imaging technique was used during TAVI prosthesis deployment to achieve optimal implantation height in relation to the non-coronary and left coronary hinge point, thereby achieving better annular sealing and lowering the risk of conduction disturbances. Precise implantation is facilitated with the prosthesis type used, because as shown, the valve has (a) specific radiopaque markers and (b) does not tend to be occlusive on LV outflow during expansion, so that there is no need to rush during prosthesis deployment because of hemodynamic instability of the patient.
However, although the valve was predilated and the prosthesis was implanted well within the aortic annulus, root angiography demonstrated significant paravalvular leakage, most likely due to incomplete expansion of the valve stent frame and consecutive mal-apposition of the prosthesis and its active sealing cuff to the heavily calcified aortic valve. As nicely shown in the case, under-expansion and mal-apposition can be successfully addressed by post-dilatation; however, it should be performed especially in the case of LVOT calcium not too aggressively, with a balloon 2-3 mm smaller in diameter than the treated anatomy, since post-dilatation in this situation with a too large-sized balloon has a higher risk of (1) aortic annular rupture, (2) injury of the prosthesis leaflets, and (3) prosthesis pop-out, especially if the prosthesis was already implanted rather high.
In summary, as highlighted by the case, successful TAVI has to be tailored to the individual patient by choosing the right prosthesis, the right implantation and post-implantation strategies, thereby balancing risks and benefits for the patient.
To download your certificate of attendance and get your CME credits, please complete the quick satisfaction survey now!
Access here





4 comments
pre-dilation and post
“The outcomes of operation, particularly in terms of PVL and the need for PPM, may be higher than those of TAVI in patients with a normal annulus. The use of off-label devices might be necessary ; however, employing on-label devices with proper oversizing and appropriate post-dilatation could lead to better long-term results.”
“The outcomes of surgery, particularly in terms of PVL and the need for PPM, may be higher than those of TAVI in patients with a normal annulus. The use of off-label devices might be necessary however, employing on-label devices with proper oversizing and appropriate post-dilatation might lead to better long-term results.”
“The outcomes of surgery, particularly in terms of PVL and the need for PPM, may be higher than those of TAVI in patients with a normal annulus. The use of off-label devices might be necessary however, employing on-label devices with proper oversizing and appropriate post-dilatation might lead to better long-term results.”