Atrial flow regulator implantation through atrial septal defect of previous mitral transcatheter edge-to-edge repair: bailout strategy
Supported by the EuroIntervention Journal
Mitral transcatheter edge-to-edge repair (M-TEER) has reshaped the prognosis for heart failure patients with secondary mitral regurgitation, but recurrence remains a real challenge when surgical options are off the table.
This case highlights an innovative use of an atrial flow regulator (AFR) to manage elevated left atrial pressure through a residual iatrogenic atrial septal defect (iASD) years after M-TEER.
Authors
Luigi Emilio Pastormerlo1, Marta Casula2, Paola Russo3, Augusto Esposito1, Massimiliano Mariani1, Giovanni Benedetti1, Giuseppe Santoro4 and Sergio Berti1.
- UOC Diagnostica Interventistica Fondazione Toscana Gabriele Monasterio Massa, Italy;
- Clinical Experimental Cardiology, Clinical and Interventional Cardiology, University of Sassari, Sassari, Italy;
- Università di Roma La Sapienza, Roma;
- UOC Cardiologia Pediatrica e del Congenito Adulto Fondazione Toscana Gabriele Monasterio Massa, Italy
Introduction
Mitral transcatheter edge-to-edge repair (M-TEER) improves prognosis of heart failure (HF) patients with secondary mitral regurgitation (MR)1. Nevertheless, some patients experience significant MR recurrence during follow-up, due to left ventricle (LV) remodeling and left atrial pressure (LAP) increase. MR recurrence is associated with HF hospitalisation and cardiac mortality2. No many options are available in this clinical setting, as surgical risk for mitral valve replacement is often prohibitive and new M-TEER frequently unfeasible.
Persistent iatrogenic atrial septal defects (iASDs) are quite common (about 40 %) after procedures that need for transeptal puncture, such as M-TEER. Prevalence and entity of these iASDs depend on follow-up time, type of procedure, and hemodynamic conditions. Persistence of iASD seems to regulate LAP with better left atrial reverse remodeling compared to patients without patent iASD3, being potentially advantageous.
Case Report
Here, we propose fixation of patent iASD by implantation of an Occlutech® atrial flow regulator 8-mm device in a patient with HFrEF and recurrence of moderate-to-severe mitral regurgitation four years after M-TEER.
Patient experienced multiple episodes of worsening HF (WHF). At last hospital admission, NT-proBNP was 3,656 ng/l, transthoracic echocardiography (TTE) revealed severe reduction of LV ejection fraction (LVEF) at 30 %, moderate-to-severe MR, and moderate tricuspid regurgitation (TR). Mean gradient across the mitral valve was 4 mmHg. A small residual iASD was visible on TTE.
Right heart catheterisation (RHC) revealed post-capillary pulmonary hypertension (18 mmHg) with normal pulmonary vascular resistance (1.6 Wood units). We decide to proceed to AFR implantation. The procedure was performed with intracardiac echocardiography (ICE) guidance and awake patient4 just after THC. ICE catheter was positioned in the right atrium, and a small residual iASD was visualised in the mid infero-posterior part of the fossa ovalis (Figure 1, boxes A and B; Video 1).
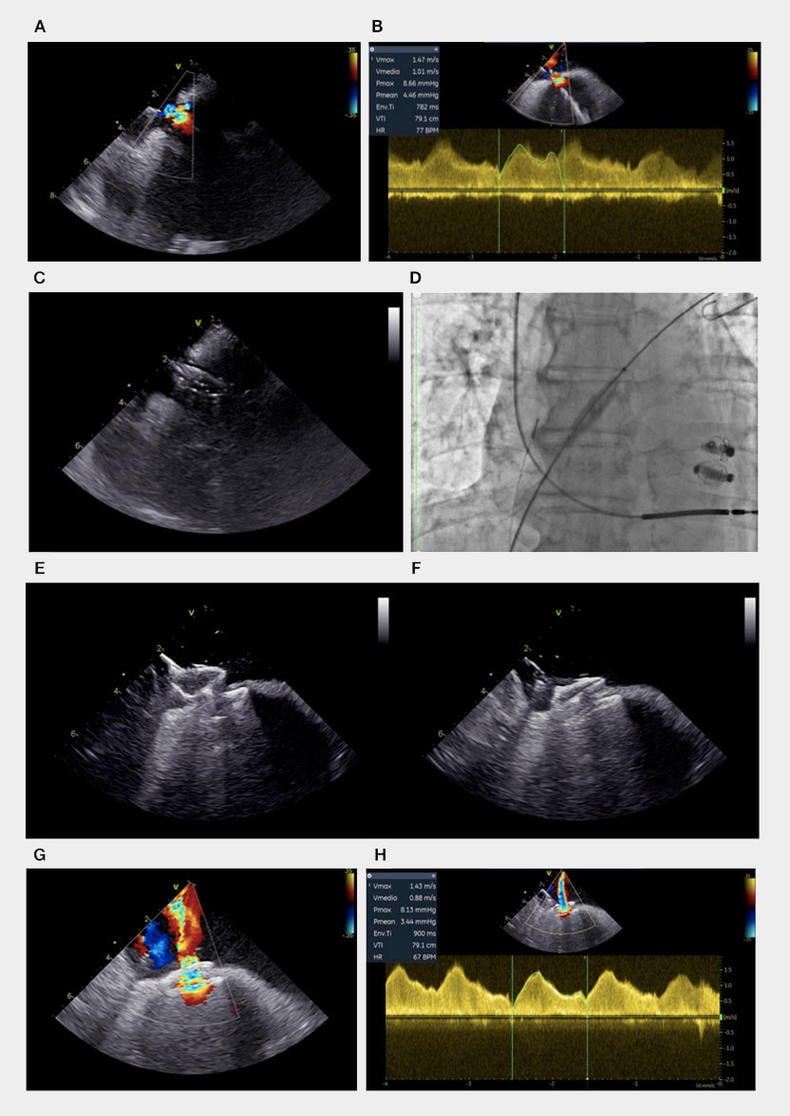
Figure 1. Different steps of intertrial shunt device implantation. ASD, ICE visualization (A) and continuous wave evaluation (B); balloon septostomy, ICE (C) and fluoroscopic visualization (D); AFR pre-release push and pull manoeuvre, ICE visualization (E-F); Final interatrial shunt, color doppler (G) and continuous wave evaluation (H).
It was quite easy to advance an idrophilic 0.035 guidewire through iASD into the left superior pulmonary vein. After exchange with a stiffer guidewire, iASD was dilated using a 12-mm balloon (Figure 1, boxes C and D; Video 2).
AFR was positioned across the septum and released after confirmation of device stability by push-and-pull manoeuvre (Figure 1, boxes E and F; Video 3).
ICE confirmed a significant improvement of left-to-right atrial shunt with a laminar flow (Figure 1, boxes G and H; Video 4).
At 90-day follow-up, patient was asymptomatic (NYHA I), transthoracic echocardiography revealed moderate MR with unchanged LVEF, no significant improvement in TR grade. Wedge pressure was 10 mmHg at RHC.
To our knowledge, this is the first report suggesting that AFR may have a favorable clinical impact on HFrEF patients with late worsening of mitral regurgitation after M-TEER.
In this specific setting, residual iASD appears as a safe and fast route to implant AFR device with the aim of reducing LAP by fixation and enlargement of iASD.
Large studies may evaluate AFR as a bailout strategy for recurrent functional MR and WHF after M-TEER.
References
- Stone GW, Abraham WT, Lindenfeld J, Kar S, Grayburn PA, Lim DS, Mishell JM, Whise-nant B, Rinaldi M, Kapadia SR, Rajagopal V, Sarembock IJ, Brieke A, Marx SO, Cohen DJ, Asch FM, Mack MJ. Five-Year Follow-up after Transcatheter Repair of Secondary Mitral Regurgitation. New England Journal of Medicine. 2023;388:2037–48.
- Sugiura A, Kavsur R, Spieker M, Iliadis C, Goto T, Öztürk C, Weber M, Tabata N, Zimmer S, Sinning J-M, Mauri V, Horn P, Kelm M, Baldus S, Nickenig G, Westenfeld R, Pfister R, Becher MU. Recurrent Mitral Regurgitation After MitraClip: Predictive Factors, Morphology, and Clinical Implication. Circ Cardiovasc Interv. 2022;15.
- Toyama K, Rader F, Kar S, Kubo S, Shiota T, Nishioka T, Siegel RJ. Iatrogenic Atrial Septal Defect After Percutaneous Mitral Valve Repair With the MitraClip System. Am J Cardiol. 2018;121:475–9.
- Pastormerlo L, Orazi F, Carapellucci E, Ferrari Chen YF, Mariani M, D’Agostino A, Bene-detti G, Santoro G, Berti S. Intracardiac Echocardiography–Guided Atrial Flow Regulator Implanta-tion for Heart Failure. JACC Cardiovasc Interv. 2025;18:668–9.
Conflicts of interest
- Dr Pastormerlo is proctor for Boston Scientific.
- Dr Santoro is proctor for Occlutech, Abbott and GORE - Italy.
- Dr Berti is proctor for Boston Scientific, Abbott, and Edwards Lifesciences.
- All other authors have reported that they have no relationships relevant to the contents of this paper to disclose.




No comments yet!