27 Mar 2023
Successful rotablation of a calcium spike blocking the motion of a mechanical prosthetic leaflet
Supported by the EuroIntervention Journal
Thanks to this EIJ image, consider the case of a 46-year-old female patient who underwent a first aortic valve replacement at the age of 25, a second one 10 years later, together with a mitral valve replacement, and who presented with a displaced calcium spike from the LVOT blocking the anteroseptal leaflet.
Authors
Yassin Belahnech1, Gerard Martí1, Laura Dos-Subirà1, Bernat Serra1, Eduard Rodenas-Alesina1, Bruno García Del Blanco1
Case summary
A 46-year-old woman with a history of congenital subaortic stenosis underwent palliative balloon valvuloplasty during childhood and subsequent surgical repair at 25 years old, that included aortic valve replacement. She later developed a severe paravalvular leak and recurrent subaortic stenosis, requiring reintervention at 35 years old. A subaortic resection was performed, along with a mechanical aortic valve replacement and an enlargement of the left ventricular outflow tract (LVOT) as per the Manoogian technique. During follow-up, she developed severe mitral stenosis and regurgitation and underwent a mitral valve replacement (On-X 25 mm, Life Technologies, Inc).
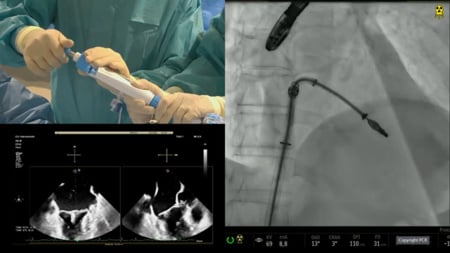
In the early postoperative period, she complained of left arm weakness and was diagnosed with a right middle cerebral artery stroke. The patient declined a transesophageal echocardiogram (TEE), but a transthoracic study (TTE) demonstrated normal prosthetic function. Despite a correct therapeutic anticoagulation range, a routine TTE one month after surgery showed a fixed anteroseptal leaflet with increased gradient with non-diagnostic TEE images for thrombus (Figures 1A and 1B).

Figure 1: 2D (Figure 1A) and 3D (Figure 1B) transesophageal ultrasound images displaying prosthetic obstruction of the anteroseptal leaflet. Figures 1C and 1D ilustrate 2D and 3D cardiac CT images of the mechanism of obstruction related to a calcium spike blocking leaflet motion. Figure 1E shows fluoroscopic image of a failed attempt to mobilize calcium spikes using a straight guidewire and the tip of the guide-catheter. Figure 1D shows a fluoroscopic image of RotaWire arteriovenous loop and ratoablation damaging the free margin of the anteroseptal leaflet. Figures 1G and 1F show by biplanar fluoroscopy the application of rotablation after removing the Rotawire, changing the orientation, minimizing damage of the leaflet, and performing a semilunar movement over the calcium spike. Figure 1I displays a follow-up 3D cardiac CT image revealing the avulsion of the calcium spike, unrestricted leaflet motion, and a small erosion of the free margin of the anteroseptal leaflet.
She was admitted and unfractionated heparin was immediately started. A synchronized cardiac computed tomography (CT) revealed a displaced calcium spike from the LVOT, blocking the anteroseptal leaflet (Figures 1C, 1D).
As the patient was a high-risk surgical candidate based on three previous sternotomies and the recent stroke, a percutaneous approach was offered. A Sentinel Cerebral Protection System (Boston Scientific, MA, USA) was deployed. Under conscious sedation and through the femoral vein, a Fast-Cath (Abbott, TX, USA) sheath was progressed and a transseptal puncture was achieved with a Brockenbrough needle, guided by intracardiac echocardiography. Mobilization of the leaflet was attempted by hitting it with a 0.035-inch straight guidewire and with the tip of 7Fr Judkins Right 4 guiding catheter (Figure 1E).
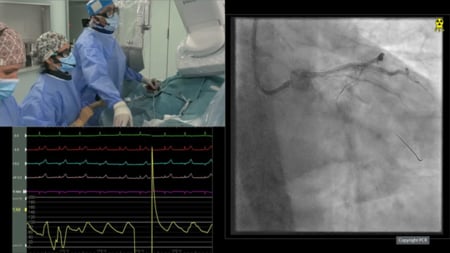
After several unsuccessful attempts, a 0.014-inch RotaWire floppy was crossed through the prosthetic valve and externalized to create a venoarterial loop. Rotational atherectomy with Rotapro (Boston Scientific, MA, USA) was performed using a 1.5 mm olive-shaped burr. However, the orientation conditioned by the venoarterial loop may damage the free margin of the leaflet (Figure 1F).
After removing the Rotawire, the orientation was changed, and lunate movements were performed to address the entire range of the calcium spike, minimizing damage of the leaflet. Complete leaflet motion was achieved after 5 minutes of burr at 180.000 r.p.m without periprocedural complications (Figure 1G, 1H and Moving image 1), and the patient was safely discharged home.
A cardiac CT one month after the procedure showed avulsion of the calcium spike, unobstructed leaflet motion, and a small erosion of the free margin of the anteroseptal leaflet (Figure 1I). TTE at one year showed normal prosthetic function.
In summary, leaflet obstruction of mechanical valves is a severe condition that may result from thrombosis or pannus formation, but other mechanisms have been reported in which multimodality imaging is crucial1,2. Although catheter manipulation of a thrombosed prosthesis has been successfully described3,4, this is the first successful report of rotablation of a displaced calcium spike blocking a mechanical prosthetic leaflet.
Supplementary data
References
- Deviri E, Sareli P, Wisenbaugh T, Cronje SL. Obstruction of mechanical heart valve prostheses: clinical aspects and surgical management. J Am Coll Cardiol. 1991 Mar 1;17(3):646-50. doi: 10.1016/s0735-1097(10)80178-0. PMID: 1993782.
- Tanis W, Habets J, van den Brink RB, Symersky P, Budde RP, Chamuleau SA. Differentiation of thrombus from pannus as the cause of acquired mechanical prosthetic heart valve obstruction by non-invasive imaging: a review of the literature. Eur Heart J Cardiovasc Imaging. 2014 Feb;15(2):119-29. doi: 10.1093/ehjci/jet127. Epub 2013 Aug 2. PMID: 23913330.
- Jabbour S, Saldinger M, Alexander JC. Hemodynamic stabilisation of acute prosthetic valve thrombosis using percutaneous manipulation. Catheter Cardiovasc Diagn. 1996;39:e314-e316.
- Hariram V. Percutaneous Management of Prosthetic Valve Thrombosis. Indian Heart J. 2014;66:427-429.
Affiliation
- Cardiology Department, Vall d’Hebron University Hospital. Vall d’Hebron Research Institute. Universitat Autònoma de Barcelona. Barcelona, Spain.
Conflict of interest statement
- Yassin Belahnech is supported by research non-conditioned grant (CM22/00242)
- Bernat Serra has consultant/speaker fees from Boston Scientific
- All other authors have reported that they have no relationships relevant to the contents of this paper to disclose




2 comments
I would like to have the authros present this caser at our cath conference in Washington Dc via the web; how can I reach the them Lowell Satler [email protected]
Hi Dr Satler, this is Bruno Garcia from Barcelona. one of us will be delighted top help or participate via web in your course or seminar, you can join us either at [email protected] or [email protected]