23 Jan 2025
Evidence base: A description of the key, so far published, studies in the space of drug coated balloons
Authored by Hector Garcia-Garcia and Jorge Sanz-Sanchez, this article examines pivotal randomized clinical trials on drug-coated balloons in coronary artery disease. It highlights their role in managing in-stent restenosis, de novo small vessel lesions, and large vessel lesions while exploring future directions through ongoing trials.

This chapter discusses the most relevant clinical data from randomized clinical trials (RCTs) evaluating the role of drug coated balloons (DCBs) for the management of coronary artery disease (CAD) with a focus on three different clinical scenarios:
- in-stent restenosis (ISR),
- de novo lesions in small vessels
- de novo lesions in large vessels.
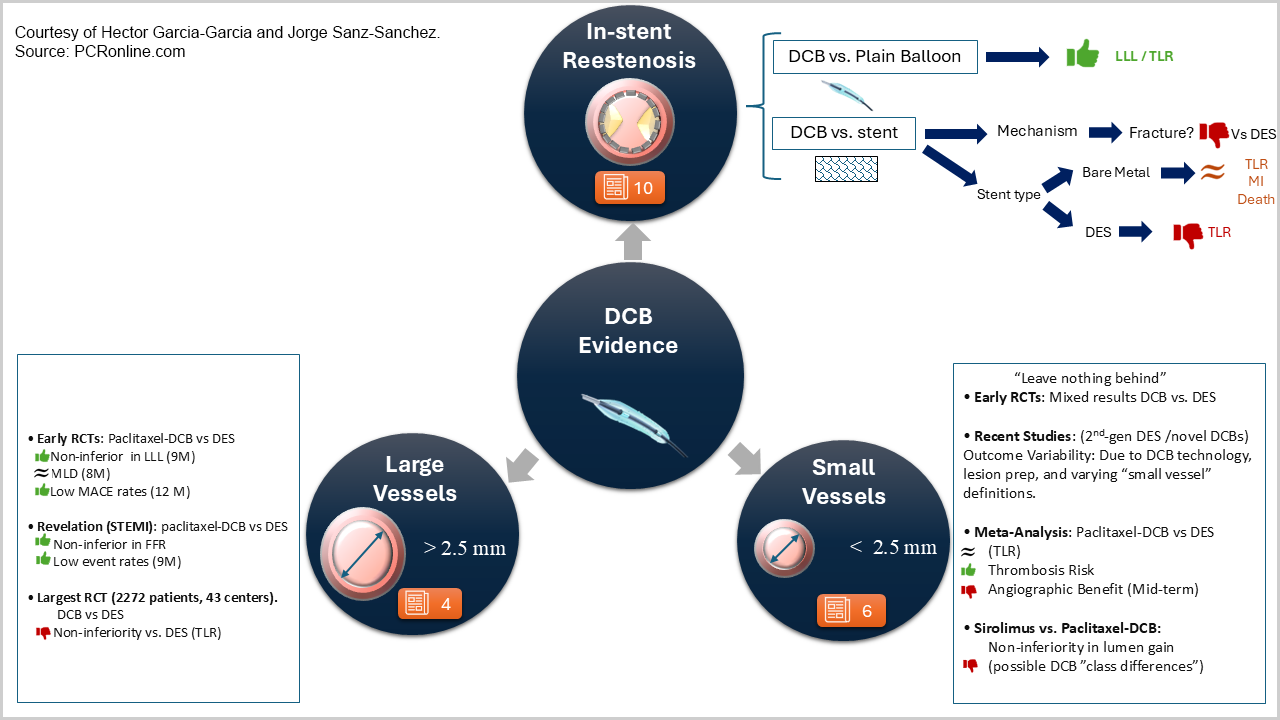
Figure 1
Courtesy of Hector Garcia-Garcia and Jorge Sanz-Sanchez. Source: PCRonline.com
We also explore the potential future directions for this therapy, drawing insights from major ongoing RCTs.
In-stent restenosis
DCBs represent an appealing alternative to drug eluting stents (DES) for patients present with ISR as it avoids the implantation of multiple layers of metal. Nowadays, ISR remains the clinical scenario with the largest amount of evidence. When compared to plain old balloon angioplasty, DCBs have shown to be superior in terms of reduced lumen loss and need for target lesion revascularization.(1) However, when compared to DES, its efficacy is determined by the initial stent type and mechanism of ISR.(2) In patients presenting with bare-metal stent restenosis, DCBs have shown similar rates of target lesion revascularization, myocardial infarction and all-cause death as compared to DES (Table 1).
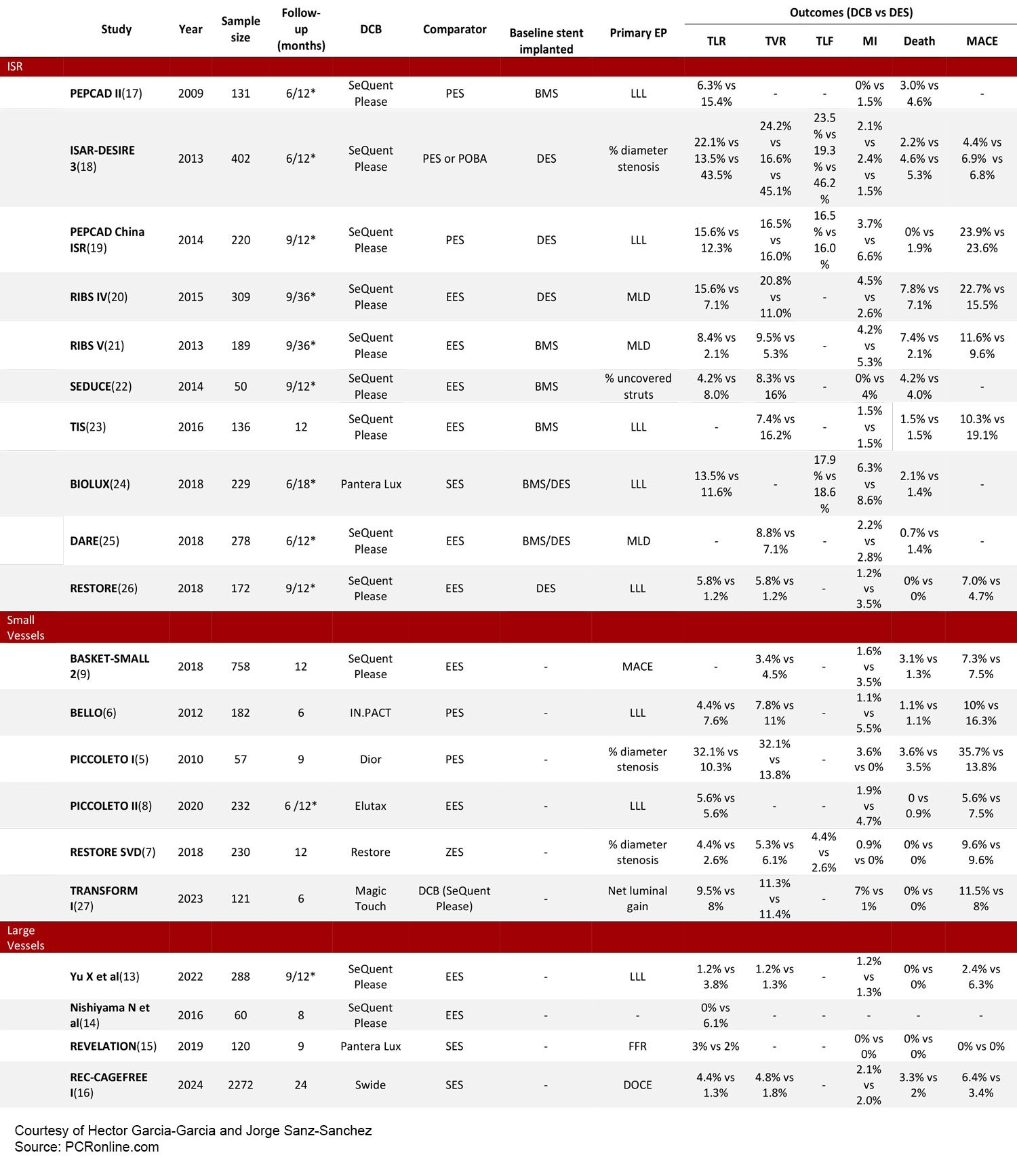
Table 1 - Clinical outcomes for Transform I reported as SCB vs PCB. *6 or 9-month follow-up for angiographic outcomes and 12 or 36 for clinical outcomes.
BMS= bare metal stent, DES= dug eluting stent, DCB= drug coated balloon, DOCE= device oriented composite endpoint, EES= everolimus eluting stent, FFR= fractional flow reserve, LLL= late luminal loss, MI= myocardial infarction, MACE= major adverse cardiac events, MLD = minimal lumen diameter , POBA= plain old balloon angioplasty, TLF= target lesion failure, TLR= target lesion revascularization, TVR= target vessel revascularization, PES= paclitaxel eluting stent, SES= sirolimus eluting stent, ZES= zotarolimus eluting stent
Courtesy of Hector Garcia-Garcia and Jorge Sanz-Sanchez. Source: PCRonline.com
Of note, intracoronary imaging is of paramount importance, as re-stenting will be required in cases of stent fracture.(3) In patients presenting with DES restenosis, repeat stenting with DES has shown to be moderately more effective than DCB in terms of TLR (Table 1), holding IA class recommendation in current guidelines.(4)
De novo lesions in small vessels
The use of DCB in de novo lesions which are not appealing for the implantation of DES (i.e. small vessels with diffuse disease) represents a growing indication for DCB, as it follows a strategy of “leaving nothing behind”. Two early RCTs reported conflicting results about the effects of DCB as compared to early-generation DES on angiographic outcomes in patients with native small vessel CAD.(5,6) More recently, RCTs with the use of second-generation DES and novel DCB devices provided new evidence about the clinical and angiographic effects of these treatments.(7–9) Differences in study results might not only be explained by variations in DCB technology and lesion preparation, but also by discrepancies in the definition of a small vessel size which varied from <3 mm and <2.8 mm to even ≤2.75 mm (Table 1).(10) Meta-analysis have shown that the use of paclitaxel-DCB is associated with risks of target vessel revascularization and restenosis that are similar to DES (OR: 0.97; 95%CI: 0.56–1.68; p=0.92; and OR: 1.12; 95%CI 0.69–1.84; p=0.64, respectively), while DCB yielded to a significant reduction in the risk of vessel thrombosis (OR: 0.12; 95%CI: 0.01–0.94; p=0.04), and DES implantation resulted in slightly better angiographic surrogate endpoints at mid-term follow-up.(11)
The only head-to-head comparison between sirolimus-DCB and paclitaxel-DCB in patients with de novo small vessel disease failed to demonstrate the non-inferiority of sirolimus DCB with regard to net lumen gain at 6 months (0.25±0.40 mm with sirolimus-DCB versus 0.48±0.37 mm with paclitaxel-DCB; p value for noninferiority=0.173), suggesting a potential lack of class effect among DCBs. (12)
De novo lesions in large vessels
Evidence supporting the safety and efficacy of DCBs in patients presenting with de novo lesions in large vessels is scarce and conflicting. Two initial small RCTs powered for angiographic outcomes have shown that paclitaxel DCB is non-inferior to DES in terms of late luminal loss at 9-month follow-up (−0.19±0.49mm vs 0.03±0.64mm, p value for noninferiority =0.019).(13) In addition, no significant differences in minimal lumen diameter between DCB and DES were found at 8 months follow-up, with low 12-month MACE rates (14)(Table 1).
The Revelation trial is the only RCT available in patients presenting with STEMI. 120 patients were randomized to paclitaxel-DCB or DES in a single center. At 9-month follow-up, a paclitaxel-DCB strategy was shown to be noninferior to DES in terms of fractional flow reserve with low clinical event rates.(15) However, in the only available RCT powered for a clinically relevant composite endpoint including cardiovascular death, target vessel myocardial infarction and target lesion revascularization, among 2272 patients in 43 centers; a strategy of DCB-PCI did not achieve non-inferiority as compared with DES driven by higher rates of TLR in the DCB group(16) (Table 1). Therefore, the role of DCB in patients with de novo lesions in large vessels is yet to be determined.
Ongoing evidence
Three large clinically powered RCTs are currently enrolling patients to elucidate the role of DCB in patients with de novo lesions in large vessels. Transform II (NCT04893291) is a multicenter noninferiority RCT comparing a sirolimus-DCB to everolimus-DES in terms of target lesion failure in patients presenting with native CAD. A total of 1325 patients with a vessel diameter between 2 mm and 3.5 mm will be enrolled. The SELUTION DeNovo Trial is a multicenter non-inferiority RCT comparing a strategy of sirolimus-DCB PCI with provisional DES to a strategy of PCI with systematic DES implantation. The primary endpoint is target vessel failure at one and five years. A total of 3162 all-comer patients with native CAD will be enrolled with the exception of those presenting with left main disease, chronic total occlusions and ST-segment elevation myocardial infarction. Finally, the COPERNICAN trial (NCT06353594) is evaluating a reduced stent strategy based on paclitaxel-DCB culprit-lesion PCI with conventional DES coronary revascularization among 1272 patients presenting with STEMI. The primary endpoint is target lesion failure at 1 year with an extended follow-up at 10 years.
References
- Giacoppo D., Gargiulo G., Aruta P., Capranzano P., Tamburino C., Capodanno D. Treatment strategies for coronary in-stent restenosis: systematic review and hierarchical Bayesian network meta-analysis of 24 randomised trials and 4880 patients. BMJ 2015;351:h5392. Doi: 10.1136/bmj.h5392.
- Korjian S., McCarthy KJ., Larnard EA., et al. Drug-Coated Balloons in the Management of Coronary Artery Disease. Circ Cardiovasc Interv 2024;17(5):e013302. Doi: 10.1161/CIRCINTERVENTIONS.123.013302.
- Stefanini GG., Alfonso F., Barbato E., et al. Management of myocardial revascularisation failure: an expert consensus document of the EAPCI. EuroIntervention 2020;16(11):e875–90. Doi: 10.4244/EIJ-D-20-00487.
- Vrints C., Andreotti F., Koskinas KC., et al. 2024 ESC Guidelines for the management of chronic coronary syndromes: Developed by the task force for the management of chronic coronary syndromes of the European Society of Cardiology (ESC) Endorsed by the European Association for Cardio-Thoracic Surgery (EACTS). Eur Heart J 2024;45(36):3415–537. Doi: 10.1093/eurheartj/ehae177.
- Cortese B., Micheli A., Picchi A., et al. Paclitaxel-coated balloon versus drug-eluting stent during PCI of small coronary vessels, a prospective randomised clinical trial. The PICCOLETO study. Heart 2010;96(16):1291–6. Doi: 10.1136/hrt.2010.195057.
- Latib A., Colombo A., Castriota F., et al. A randomized multicenter study comparing a paclitaxel drug-eluting balloon with a paclitaxel-eluting stent in small coronary vessels: the BELLO (Balloon Elution and Late Loss Optimization) study. J Am Coll Cardiol 2012;60(24):2473–80. Doi: 10.1016/j.jacc.2012.09.020.
- Tang Y., Qiao S., Su X., et al. Drug-Coated Balloon Versus Drug-Eluting Stent for Small-Vessel Disease: The RESTORE SVD China Randomized Trial. JACC Cardiovasc Interv 2018;11(23):2381–92. Doi: 10.1016/j.jcin.2018.09.009.
- Cortese B., Di Palma G., Guimaraes MG., et al. Drug-Coated Balloon Versus Drug-Eluting Stent for Small Coronary Vessel Disease: PICCOLETO II Randomized Clinical Trial. JACC Cardiovasc Interv 2020;13(24):2840–9. Doi: 10.1016/j.jcin.2020.08.035.
- Jeger R V., Farah A., Ohlow M-A., et al. Drug-coated balloons for small coronary artery disease (BASKET-SMALL 2): an open-label randomised non-inferiority trial. Lancet 2018;392(10150):849–56. Doi: 10.1016/S0140-6736(18)31719-7.
- Sanz-Sánchez J., Chiarito M., Gill GS., et al. Small Vessel Coronary Artery Disease: Rationale for Standardized Definition and Critical Appraisal of the Literature. Journal of the Society for Cardiovascular Angiography & Interventions 2022;1(5). Doi: 10.1016/j.jscai.2022.100403.
- Sanz Sánchez J., Chiarito M., Cortese B., et al. Drug-Coated balloons vs drug-eluting stents for the treatment of small coronary artery disease: A meta-analysis of randomized trials. Catheter Cardiovasc Interv 2020. Doi: 10.1002/ccd.29111.
- Kai N., W SP., Antonio C., et al. A Prospective Randomized Trial Comparing Sirolimus-Coated Balloon With Paclitaxel-Coated Balloon in De Novo Small Vessels. JACC Cardiovasc Interv 2023;16(23):2884–96. Doi: 10.1016/j.jcin.2023.09.026.
- Yu X., Wang X., Ji F., et al. A Non-inferiority, Randomized Clinical Trial Comparing Paclitaxel-Coated Balloon Versus New-Generation Drug-Eluting Stents on Angiographic Outcomes for Coronary De Novo Lesions. Cardiovasc Drugs Ther 2022;36(4):655–64. Doi: 10.1007/s10557-021-07172-4.
- Nishiyama N., Komatsu T., Kuroyanagi T., et al. Clinical value of drug-coated balloon angioplasty for de novo lesions in patients with coronary artery disease. Int J Cardiol 2016;222:113–8. Doi: 10.1016/j.ijcard.2016.07.156.
- Vos NS., Fagel ND., Amoroso G., et al. Paclitaxel-Coated Balloon Angioplasty Versus Drug-Eluting Stent in Acute Myocardial Infarction: The REVELATION Randomized Trial. JACC Cardiovasc Interv 2019;12(17):1691–9. Doi: 10.1016/j.jcin.2019.04.016.
- Gao C., He X., Ouyang F., et al. Drug-coated balloon angioplasty with rescue stenting versus intended stenting for the treatment of patients with de novo coronary artery lesions (REC-CAGEFREE I): an open-label, randomised, non-inferiority trial. The Lancet 2024;404(10457):1040–50. Doi: 10.1016/S0140-6736(24)01594-0.
- Unverdorben M., Vallbracht C., Cremers B., et al. Paclitaxel-Coated Balloon Catheter Versus Paclitaxel-Coated Stent for the Treatment of Coronary In-Stent Restenosis. Circulation 2009;119(23):2986–94. Doi: 10.1161/CIRCULATIONAHA.108.839282.
- Byrne RA., Neumann F-J., Mehilli J., et al. Paclitaxel-eluting balloons, paclitaxel-eluting stents, and balloon angioplasty in patients with restenosis after implantation of a drug-eluting stent (ISAR-DESIRE 3): a randomised, open-label trial. The Lancet 2013;381(9865):461–7. Doi: https://doi.org/10.1016/S0140-6736(12)61964-3.
- Xu B., Qian J., Ge J., et al. Two-year results and subgroup analyses of the PEPCAD China in-stent restenosis trial: A prospective, multicenter, randomized trial for the treatment of drug-eluting stent in-stent restenosis. Catheterization and Cardiovascular Interventions 2016;87(S1):624–9. Doi: https://doi.org/10.1002/ccd.26401.
- Alfonso F., Pérez-Vizcayno MJ., Cuesta J., et al. 3-Year Clinical Follow-Up of the RIBS IV Clinical Trial: A Prospective Randomized Study of Drug-Eluting Balloons Versus Everolimus-Eluting Stents in Patients With In-Stent Restenosis in Coronary Arteries Previously Treated With Drug-Eluting Stents. JACC Cardiovasc Interv 2018;11(10):981–91. Doi: https://doi.org/10.1016/j.jcin.2018.02.037.
- Alfonso F., Pérez-Vizcayno MJ., García del Blanco B., et al. Long-Term Results of Everolimus-Eluting Stents Versus Drug-Eluting Balloons in Patients With Bare-Metal In-Stent Restenosis: 3-Year Follow-Up of the RIBS V Clinical Trial. JACC Cardiovasc Interv 2016;9(12):1246–55. Doi: https://doi.org/10.1016/j.jcin.2016.03.037.
- Adriaenssens T., Dens J., Ughi GJ., et al. Optical coherence tomography study of healing characteristics of paclitaxel-eluting balloons vs. everolimus-eluting stents for in-stent restenosis: the SEDUCE (Safety and Efficacy of a Drug elUting balloon in Coronary artery rEstenosis) randomised clinical trial. EuroIntervention 2014;10(4):439–48. Doi: 10.4244/EIJV10I4A77.
- Pleva L., Kukla P., Kusnierova P., Zapletalova J., Hlinomaz O. Comparison of the Efficacy of Paclitaxel-Eluting Balloon Catheters and Everolimus-Eluting Stents in the Treatment of Coronary In-Stent Restenosis. Circ Cardiovasc Interv 2016;9(4):e003316. Doi: 10.1161/CIRCINTERVENTIONS.115.003316.
- Jensen C., Richardt G., Tölg R., et al. Angiographic and clinical performance of a paclitaxel-coated balloon compared to a second-generation sirolimus-eluting stent in patients with in-stent restenosis: the BIOLUX randomised controlled trial. EuroIntervention 2018;14(10):1096–103. Doi: 10.4244/EIJ-D-17-01079.
- Baan J., Claessen BE., Dijk KB., et al. A Randomized Comparison of Paclitaxel-Eluting Balloon Versus Everolimus-Eluting Stent for the Treatment of Any In-Stent Restenosis: The DARE Trial. JACC Cardiovasc Interv 2018;11(3):275–83. Doi: https://doi.org/10.1016/j.jcin.2017.10.024.
- Wong YTA., Kang D-Y., Lee JB., et al. Comparison of drug-eluting stents and drug-coated balloon for the treatment of drug-eluting coronary stent restenosis: A randomized RESTORE trial. Am Heart J 2018;197:35–42. Doi: https://doi.org/10.1016/j.ahj.2017.11.008.
- Ono M., Kawashima H., Hara H., et al. A Prospective Multicenter Randomized Trial to Assess the Effectiveness of the MagicTouch Sirolimus-Coated Balloon in Small Vessels: Rationale and Design of the TRANSFORM I Trial. Cardiovasc Revasc Med 2021;25:29–35. Doi: 10.1016/j.carrev.2020.10.004.
The Essentials - Drug-Coated Balloons
Authors




