17 Nov 2025
Validation of intravascular ultrasound-defined optimal stent expansion criteria for favorable 1-year clinical outcomes
Selected in JACC: Cardiovascular Interventions by R. Sava , N. Ryan
This individual patient data analysis of the three largest RCTs evaluating IVUS-guided stent optimisation showed that an MSA > 5.5 mm2 was associated with reduced TVF at one year.
References
Authors
Sang-Hyup Lee, Xiaoping Jin, Yong-Joon Lee, Jing Kan, Zhen Ge, Seung-Jun Lee, Sung-Jin Hong, Chul-Min Ahn, Jung-Sun Kim, Byeong-Keuk Kim, Young-Guk Ko, Donghoon Choi, Yangsoo Jang, Gregg W. Stone, Gary S. Mintz, Shao-Liang Chen, and Myeong-Ki Hong
Reference
JACC Journals › JACC: Interventions › Archives › Vol. 18 No. 18
Published
22 September 2025
Link
Read the abstractReviewers
Our Comment
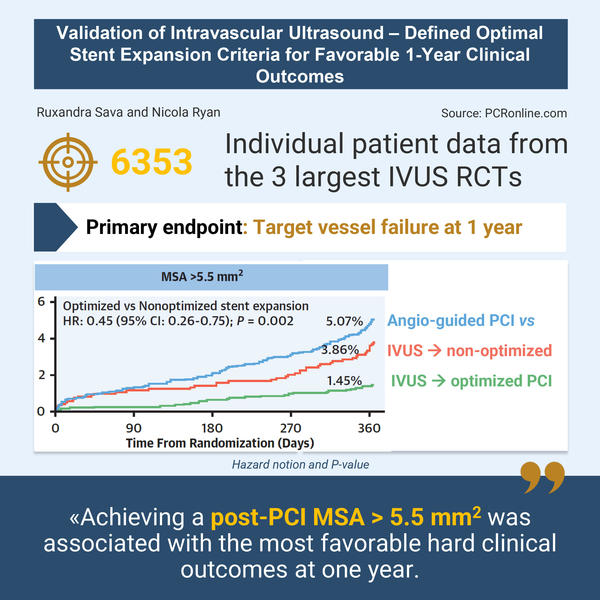
Designed by Ruxandra Sava and Nicola Ryan. Source: PCRonline.com
Why this study – the rationale/objective?
This study aimed to validate previously proposed intravascular ultrasound (IVUS)–defined optimal stent expansion criteria against hard clinical endpoints at one year, using pooled individual patient data from three randomised clinical studies, using contemporary drug eluting stents (DES).
How was it executed? The methodology
Pooled individual patient data:
The authors analysed the pooled individual patient data from three RCTs: IVUS-XPL1, ULTIMATE2 and IVUS-ACS3.
IVUS-XPL enrolled 1,400 patients from Korea, ULTIMATE 1,448 patients from China, and IVUS-ACS 3,505 patients from China, Pakistan, the UK and Italy.
Stratification
Patients treated with PCI without IVUS guidance were included in the Angiography-guided group.
Patients treated with IVUS-guided PCI were allocated to the Optimised or Non-optimised group, based on the presence or absence of each of the following stent optimisation criteria:
- Absolute expansion criteria: MSA > 5.5 mm2 and MSA > 5.0 mm2
- Relative expansion criteria:
- MSA > 100 %, or > 90 % or > 80 % of distal reference luminal area
- MSA > 90 % or > 80 % of average reference luminal area.
The group allocation was dynamic, and each patient could fall into the Optimised or Non-optimised group as a function of which expansion criteria was evaluated.
Study endpoints
- The primary endpoint was target vessel failure (TVF), a composite outcome of cardiac death, target vessel myocardial infarction (TV-MI) and target vessel revascularisation at 1 year post PCI. It should be noted that this was the original primary endpoint of the three RCTs included.
- The secondary endpoints were cardiac death and the composite of cardiac death or TV-MI.
What is the main result?
The pooled data set comprised 6,353 patients, out of which 3,208 were treated by angiography-guided PCI, 3,082 by IVUS-guided PCI and 63 patients were excluded due to insufficient data.
Primary outcome
- Patients undergoing PCI with IVUS-guidance had a lower incidence of TVF than those in the Angiography-guided group, regardless of whether they met IVUS optimisation criteria or not.
- When considering the absolute expansion criteria of MSA > 5.5 mm2, patients with an IVUS optimised procedure had a significantly lower rate of events than non-optimised patients (1.45 % vs 3.86 %; adjusted HR: 0.45; 95 % CI: 0.26-0.75; P = 0.002).
- However, achievement of an MSA > 5.0 mm2 was not associated with a reduction in TVF (1.95 % vs 3.58 %; adjusted HR: 0.68; 95 % CI: 0.41-1.14; P = 0.145).
- None of the relative stent expansion criteria were associated with benefits in terms of TVF between the Optimised and Non-optimised PCI groups.
Secondary outcome
- Patients with an IVUS-optimised procedure according to either one of the absolute expansion criteria had a reduced rate of secondary outcomes when compared to non-optimised patients.
- In contrast, non-optimised patients presented no outcome benefit by comparison to Angiography-guided PCI.
Critical reading and the relevance for clinical practice:
This individual patient data analysis of the three largest RCTs evaluating IVUS-guided stent optimisation showed that an MSA > 5.5 mm2 was associated with reduced TVF at one year. Under the assumption of lumen sphericity, this MSA corresponds with a luminal diameter of 2.6 mm. In patients from the Angiography-guided group, the post-PCI minimal luminal diameter assessed by quantitative coronary angiography that discriminates between patients with and without TVF was 2.92 mm (HR: 0.59; 95 % CI: 0.40-0.89; P = 0.010).
When examining the pre-PCI quantitative coronary angiography data amongst patients with an MSA > 5.5 mm2, non-optimised patients and those without IVUS guidance, we see that patients in the optimised group had a numerically higher reference vessel diameter than those in the non-optimised group (3.01 vs 2.63), whilst the reference vessel diameter calculated in the Angiography-guided group fell in the middle (2.89 mm). This variable was not included amongst those accounted for by the regression models and may introduce significant bias. Further limitations include a bias towards an Asian population, as generalisability to larger habitus Europeans may be somewhat limited.
Taken together, this data paints a clear picture: absolute stent size, quantified by an MSA of > 5.5 mm2, or by a diameter of > 2.92 mm as assessed by quantitative coronary angiography, are the most important predictors of reduced TVF at 1 year. One may further hypothesise regarding the pathophysiological substrate: a diameter between 2.6 and 2.9 mm may represent the mechanical limit at which sheer stress and flow currents within a stent become insufficient to prevent adverse stent outcomes such as restenosis or thrombosis.
Moreover, even in the IVUS-guided group, an MSA > 5.5 mm2 was achieved in only 61 % of patients. This adds to the body of evidence that patients with target lesions in larger vessel segments may draw more benefit from stenting than those where stenting is aimed at smaller vessel segments, where other therapies such as drug coated balloons may offer better results. This notion is particularly relevant with the positive 1-year results of the SELUTION DeNovo trial, in which the Selution sirolimus-eluting DEB was shown to be non-inferior to DES, in target arteries measuring between 2.0 and 5.0 mm4.
In conclusion, this study reinforces the use of an MSA > 5.5 mm2 to guide IVUS optimisation of coronary stenting, and suggests that, in vessels assessed to be < 2.92 mm by quantitative coronary angiography, alternatives to stents such as drug eluting ballons may be better suited to achieve optimal outcomes.
References
- Hong SJ, Kim BK, Shin DH, et al. Effect of Intravascular Ultrasound-Guided vs Angiography-Guided Everolimus-Eluting Stent Implantation: The IVUS-XPL Randomized Clinical Trial. JAMA. 2015;314(20):2155-2163. doi:10.1001/jama.2015.15454
- Zhang J, Gao X, Kan J, et al. Intravascular Ultrasound Versus Angiography-Guided Drug-Eluting Stent Implantation: The ULTIMATE Trial. J Am Coll Cardiol. 2018;72(24):3126-3137. doi:10.1016/j.jacc.2018.09.013
- Li X, Ge Z, Kan J, et al. Intravascular ultrasound-guided versus angiography-guided percutaneous coronary intervention in acute coronary syndromes (IVUS-ACS): a two-stage, multicentre, randomised trial. Lancet. 2024;403(10439):1855-1865. doi:10.1016/S0140-6736(24)00282-4
- 1-year SELUTION DeNovo results: drug-eluting balloon strategy vs. systematic drug-eluting stent in de novo coronary disease







1 comment
Excellent Ivus guided pci less tvf