Amulet IDE: Amplatzer Amulet Left Atrial Appendage Occluder RCT - 12-Month Closure Follow-up
Reported from TCT 2021
Ali Nazmi Calik reviews Amulet IDE, a prospective, randomized, multi-center and active-control trial designed to compare the Amulet and Watchman 2.5 devices in a head-to-head fashion, and presented by Dhanunjaya Lakkireddy during TCT 2021.
Why This Study? – The Rationale/Objective
The Amulet IDE trial, which was announced as a Hot Line in the ESC Congress 2021, has shown that the Amplatzer Amulet left atrial appendage (LAA) occluder device was superior for LAA occlusion at 45 days and non-inferior in terms of efficacy (preventing ischemic stroke or systemic embolism through 18 months) and safety (composite of procedure-related complications, all-cause death, major bleeding at 12 months) compared to Watchman 2.5 device.
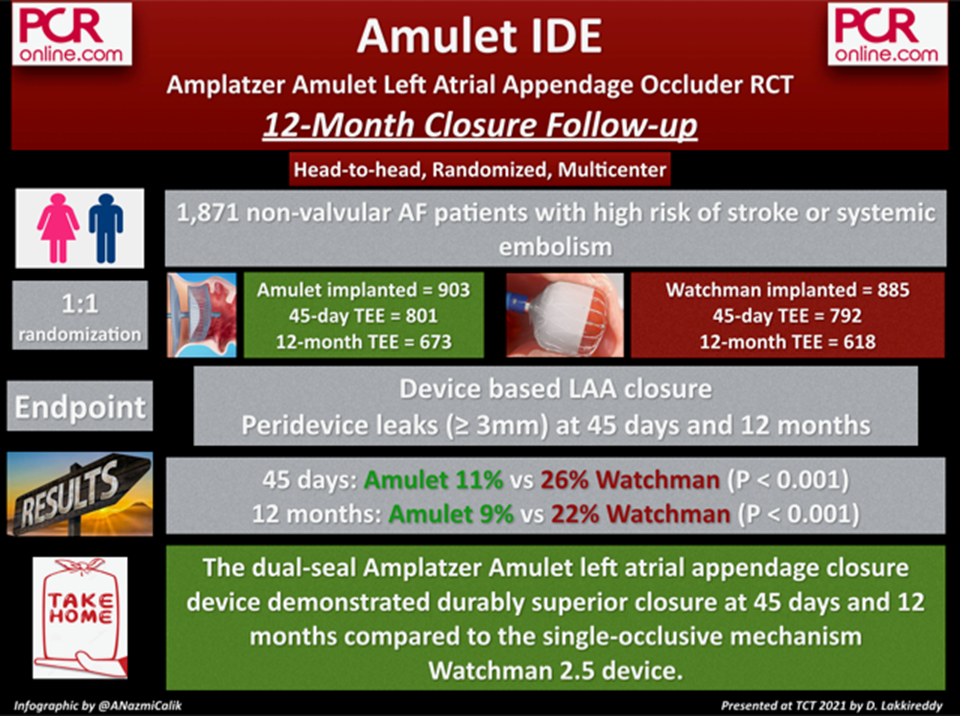
Since the limited long-term data exists on peridevice leaks (PDL), this analysis aimed to provide longitudinal data on PDLs in the Amulet and Watchman devices from the Amulet IDE trial, which is the largest RCT on LAA occlusion.
How was it executed? – The methodology
The Amulet IDE is a prospective, randomized, multi-center and active-control trial designed to compare the Amulet and Watchman 2.5 devices in a head-to-head fashion. In this further analysis of the trial, the PDLs were evaluated with transesophageal echocardiography (TEE) at 45 days and 12 months. PDLs ≥ 3 mm were defined as moderate.
What is the main result?
Analysis of the AMULET IDE Trial 1Y-ear Results
A total of 801 patients with Amulet device and 792 patients with Watchman device were assessed with TEE at 45 days. These numbers declined to 673 for Amulet and 612 for Watchman at 12 months. The superiority of the Amulet in terms of the percentage of patients with moderate peridevice leaks (≥ 3 mm) seen at 45 days (11% vs 26% with the Watchman 2.5) was maintained at 1 year (9% vs 22%; P < 0.001, for both).
As for leak progression from 45 days to 1 year, a higher number of increased PDL was observed in the Watchman group. Among patients with ≥ moderate PDL at 45 days, more Watchman (94) than Amulet (35) patients remained with ≥ moderate PDL at 12 months.
Critical reading and the relevance for the clinical practice
The dual-seal Amplatzer Amulet left atrial appendage closure device demonstrated durably superior closure at 45 days and 12 months compared to the single-occlusive mechanism Watchman 2.5 device.




No comments yet!