TRISCEND: six-month outcomes of transfemoral tricuspid valve replacement in patients with TR - a written review
Reported from TCT 2021
Background
- Tricuspid regurgitation (TR) impacts outcomes and affects a growing population of patients, especially those at advanced age.
- More than 6 % of the individuals aged 75 years old or more have moderate or greater TR.
- Isolated tricuspid surgery has been associated with unchanging in-hospital mortality of about 10 %.
- Transcatheter approaches, in particular valve replacement, represent a promising treatment option for patients who are not appropriate for surgery.
The TRISCEND Study
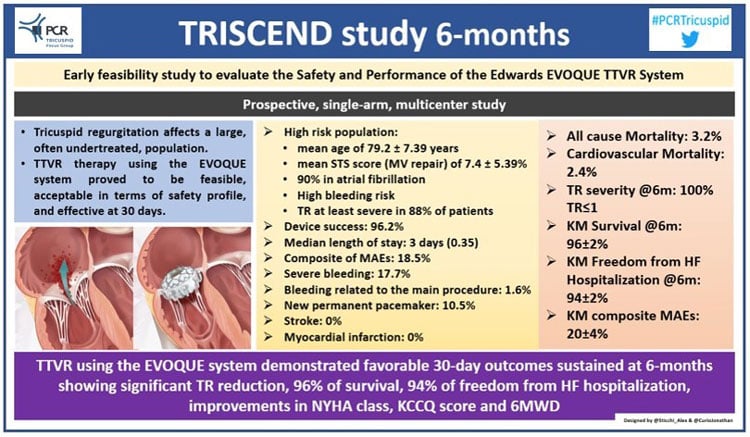
The 6-month follow-up of the TRISCEND study has been presented during the TCT Congress 2021 by principal investigator Dr. Susheel Kodali. The study is a multi-center, prospective, core lab-adjudicated, single-arm, early feasibility study designed to assess the safety and feasibility of TTVR using the EVOQUE valve (Edwards Lifesciences).
View this related interview about the TRISCEND results with Susheel Kodali
Patient population
The study population consists of 132 patients with a mean age of 79.2 ± 7.39 years and a mean STS score (MV repair) of 7.4 ± 5.39 %. Atrial fibrillation was present in 90 %, ascites in 20 %, along with several other comorbidities. TR was at least severe in 88 % of patients (43 % with massive or torrential TR).
Following the inclusion criteria, patients affected by functional or degenerative TR at least moderate and still symptomatic despite optimal medical therapy were evaluated by the local Heart Team. The patients were assessed for anatomical suitability for EVOQUE implantation using transoesophageal echocardiography and computed tomography.
Endpoints
The endpoints were device and procedural success, TR reduction, and a composite of major adverse events (MAEs: cardiovascular mortality, myocardial infarction, stroke, renal complications requiring unplanned dialysis or renal replacement therapy, severe bleeding [MVARC definition], major extensive site and vascular complications non-elective tricuspid valve re-intervention [percutaneous or surgical], major cardiac structural complications, device-related pulmonary embolism). Device success was defined as successful valve deployment and delivery system retrieval. Procedural success was defined as successful implantation of the device and absence of significant PVL at discharge.
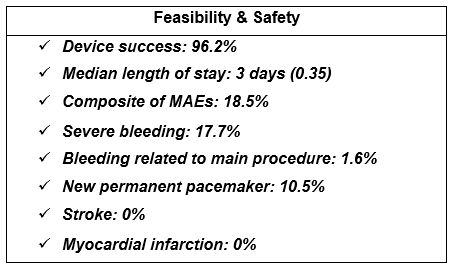
Feasibility and Safety
The study reported a device success of 96.2 % (128/133), a median length of stay of three days, and 88.4 % of patients (114/129) were discharged to home without services or nursing facilities.
The composite of MAEs was 18.5 % (23/124) with the majority of events being severe but neither life-threatening nor fatal bleedings (defined following the MVARC criteria). Among these bleeding events, two (1.6 %) were vascular access site complications, while the remaining events were not related to the procedure.
The all-cause mortality rate at 6 months was 3.2 % (4/124). Cardiovascular mortality was identified in 2.4 % (2/124) of the patients. No stroke or myocardial infarction was reported. The rate of new permanent pacemaker was 10.5 % (8/76).
Courtesy of Alessandro Sticchi
Effectiveness
Looking at the EVOQUE valve performance, there was a significant TR reduction (by Core-lab evaluation) with 100 % of patients with mild or no TR at 6 months, and 51 % with none or trace TR.
One concern of the investigators was RV failure after valve implantation, thus, patients with severe RV dysfunction were excluded. Significant worsening of the RV function was observed in one-fourth of the patients between the procedure and discharge. However, this rate decreased to 4.5 % at 30 days due to function recovery in the large majority of the affected patients.
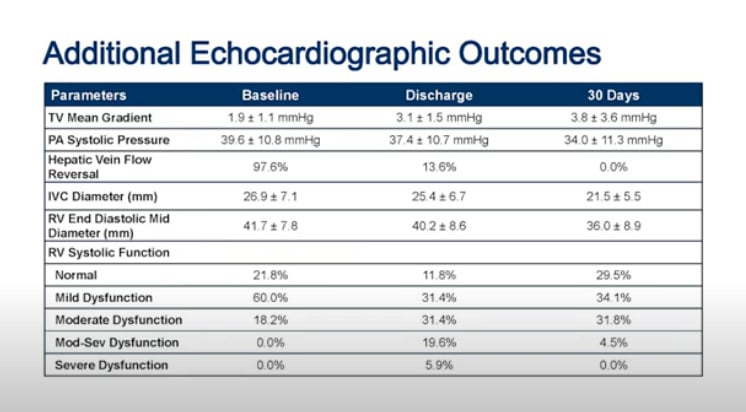
Reference: ACC21. TRISCEND: 30-day Outcomes of Transfemoral Tricuspid Valve Replacement in Patients With Tricuspid Regurgitation. Dr Susheel K. Kodali
In addition, improvement in the six-minute walking distance by 56 meters (baseline 199.5 meters) was observed. The KCCQ score improved by 27 points, and 89% of the population was in NYHA Class I-II, compared to around 20% at baseline.
Conclusions
In conclusion, TTVR using the EVOQUE valve confirmed to be safe, feasible, and effective at 6 months with sustained TR reduction and continuous clinical benefit.
Based on these encouraging results the TRISCEND II study (NCT04482062) has been initiated and it is currently ongoing. This is a prospective, multi-center, randomized controlled pivotal clinical trial evaluating the safety and effectiveness of the EVOQUE TTVR system on top of OMT compared to OMT alone in the treatment of patients with severe TR.
This trial is a glimpse into the future and further data are needed to confirm the promises of the system for the untreated TR population.
Take home messages and future research
Courtesy of Alessandro Sticchi
- New evidence supports the use of replacement devices while data accumulate concerning repair systems that are already commercially available.
- Transcatheter tricuspid valve replacement (TTVR) may offer streamlined procedures and abolition of TR in a high proportion of patients.
- Impact of TR abolition on RV function needs to be investigated.
- The appropriate anticoagulation regimen is not yet known and needs to be studied.
- Conduction disorders and PM implantation following TTVR require further analyses.
- Study enrollment “after optimized GDMT” may lead to late referral, while the new 2021 European Guidelines on the treatment of valvular heart disease emphasizes that medical optimization should not delay surgical or transcatheter treatment.
Authors






No comments yet!