Comparison of a precision care strategy with usual testing to guide management of stable patients with suspected CAD: the PRECISE randomized trial
Reported from AHA 2022
Alex Sticchi provides his take on PRECISE, which was presented during AHA 2022 in Chicago.

PICOT of the PRECISE trial - Designed by Alex Sticchi
Why this study – the rationale/objective?
The diagnostic approach to patients with new onset of stable chest pain is poorly defined. Investigations for suspected coronary artery disease (CAD) can carry on avoidable risks and increase costs without the promise to guarantee efficient care and solve patient concerns.
In this scenario, two recent studies, the Scottish Computed Tomography of the HEART (SCOT HEART) and PROspective Multicenter Imaging Study for Evaluation of chest pain (PROMISE) trials reported the successful use of coronary computed tomographic angiography (CTA) as first test routine in the assessment of suspected CAD in patients presenting with stable chest pain1,2.
The data about CTA allowed to include it as a first-line investigation in the American and European Guidelines for patients with this risk profile3–5. Moreover, the recent 2021 ACC/AHA Guideline for the Evaluation and Diagnosis of Chest pain promoted the CTA in Class 1A and as a first-line diagnostic test for intermediate-risk patients with stable chest pain with a further assessment using FFR-CT for stenosis from 40 % to 90 % in a proximal or middle coronary artery (class 2a)4.
However, CTA is not considered the established preferred diagnostic strategy among physicians, and randomized trials are needed to determine the best care pathway from the validation of the screening power of a pre-test risk score to the safety and efficacy of the test (e.g. CTA) in its potential modalities (e.g. fractional flow reserve [FFR-CT], myocardial CT perfusion).
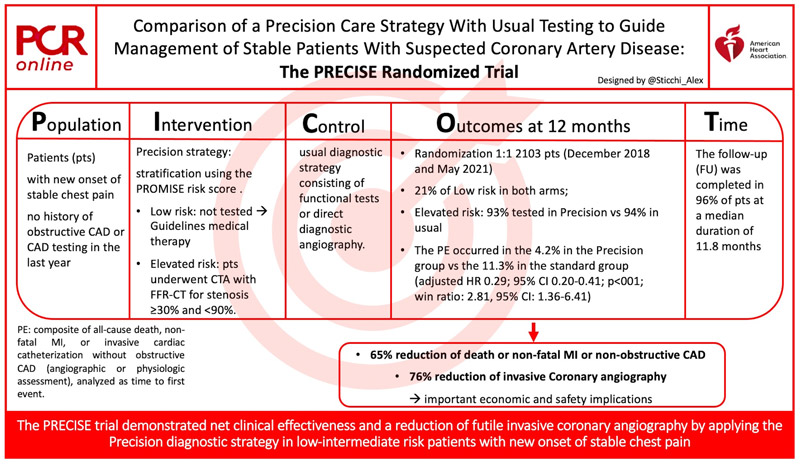
The Prospective Randomized Trial of the Optimal Evaluation of Cardiac Symptoms and Revascularization (PRECISE) trial (NCT03702244) tested a “Precision Strategy” in stable, symptomatic patients with suspected CAD compared to a standard usual strategy in terms of outcomes.
How was it executed the methodology?
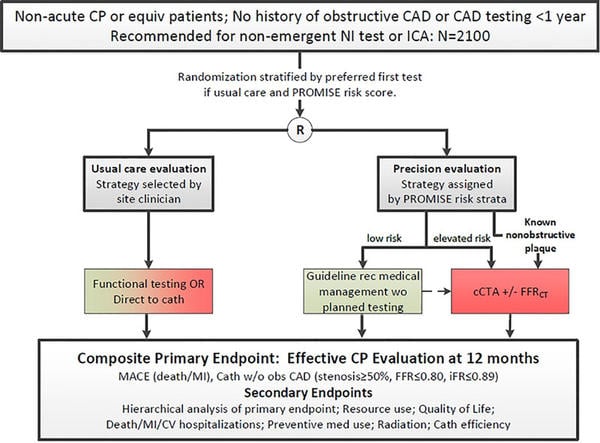
The study included patients with no history of obstructive CAD or CAD testing in the last year, presenting non-acute chest pain or equivalent symptoms. Patients were randomized to “Precision diagnostic strategy” or a usual diagnostic strategy consisting of functional tests or direct diagnostic angiography.
Before the randomization, patients were blinded stratified using the PROMISE risk score, and a preferred first test was chosen by clinicians in case of usual strategy.
In the Precision strategy, patients were assigned to a low- or elevated-risk group through the PROMISE risk score to low or elevated risks. The low-risk group was not tested and continued with only medical therapy according to guidelines. The elevated-risk group underwent CTA with selective fractional flow reserve (FFR-CT) for any stenosis meeting a threshold of ≥ 30 % and < 90 %.
The primary endpoint is a composite of all-cause death, non-fatal MI, or invasive cardiac catheterization without obstructive CAD (angiographic or physiologic assessment), analyzed as time to first event.
Several secondary endpoints included hierarchical analysis of the primary endpoint, medical costs, Quality of life (QoL), hospitalizations, proportion of invasive coronary angiogram, and revascularization within 6 months of enrollment.
All endpoint events were adjudicated by a blinded Clinical Events Committee.
Reaching a sample size of 2,100 patients provided ≥ 90 % power to detect a 35 % reduction in primary endpoint.
The statistical team was independent and had full access to the complete data.
The trial was funded by HeartFlow Inc. to assess the HeartFlow FFRCT Analysis.
From Nanna et al. American Heart Journal. Volume 245, March 2022, Pages 136-148. https://doi.org/10.1016/j.ahj.2021.12.004
What is the main result?
Prof. Pamela S. Douglas (Duke University and Clinical Research Institute) presented the trial results at the AHA 2022.
The study randomized 1:1 2,103 patients enrolled between December 2018 and May 2021 at 65 sites in US, Canada, and Europe.
The low risk was 21 % in each arm. As dictated, the low risk in the precision strategy had no testing for the majority. On the contrary, those with a high risk in both groups were tested for 93 % in the experimental group, and in 94 % in the usual test group. Per protocol, 92 % had to be tested in this elevated-risk group.
The follow-up (FU) was completed in 96 % at a median duration of 11.8 months, and all patients were included in the analysis.
Patients had a mean age of 58 (trial arm mean age: 58.0 ± 11.5 years versus [vs] usual: 58.9 ± 11.6 years), 50 % of women (trial arm: 48 % women vs usual: 52 % women), mild differences in diabetics (trial arm diabetics: 17 % vs usual 19 %). The pre-test probability was intermediate (16 in both groups), and the ASCVD (Atherosclerotic Cardiovascular Disease) 10 years was about 8.
Chest pain was the predominant symptom in 83 %, with around 25 % with typical angina.
In the Precision strategy 48 % of patients underwent CTA and 31% underwent FFR-CT.
In the usual strategy, patients underwent in:
- 32 % SPECT/PET
- 30 % Stress Echo
- 11 % Treadmill ECG
- 10 % Stress cMR
- 10 % Directly invasive coronary angiography
The primary endpoint occurred in the 4.2 % in the Precision group vs the 11.3 % in the standard group (adjusted HR 0.29; 95 % CI 0.20-0.41; p < 001; win ratio: 2.81, 95 % CI: 1.36-6.41), driven by the high rate of catheterization in the usual strategy (2.6 % vs 10.2 %; adjusted HR 0.18; 95 % CI 0.12-0.30) but without differences between the groups in all-cause death or nonfatal MI.
No death or MI events occurred in the Precision strategy deferred group (not tested).
Moreover, in the Precision strategy, a lower number of tests were performed (precision 84 % vs usual 93 %) and contemporary, more positive tests were reported (Precision 18 % vs 13 % usual strategy, p<0.001).
Other interesting findings were:
- the catheterizations were fewer in the Precision strategy
- the rates of catheterizations with non-obstructive disease in the usual testing was three times higher than in the precision strategy
- the rates of catheterization without revascularization was two times higher than the precision strategy
- the rate of revascularization was slightly higher in the Precision strategy (same for CABG).
Among the secondary endpoints, a statistically significant greater use of lipid-lowering and antiplatelet medications was found at 12 months in the Precision group.
Finally, no differences occurred in reporting angina between the two strategies at 12 months.
Limitations
- The PRECISE trial reported the real-world practice in terms of care pathway (risk-stratification, deferring testing, use of CTA/FFR-CT), and the cumulative effects of these actions cannot be evaluated separately.
- The pragmatic trial design didn’t allow mixing tests or only close monitoring in the usual strategy.
- The duration of the trial is limited to 12 months.
- The assessment of low-risk patients’ outcomes and cost analysis need to be reported separately and evaluated in dedicated randomized sub-trials.
Critical reading and the relevance for clinical practice
The PRECISE trial reached its Primary Endpoint showing a reduction of 70 % in the composite of all-cause death, non-fatal MI, or catheterization without obstructive CAD in the Precision strategy compared to usual testing at the 12 months FU.
No differences occurred in all-cause of death, MI, and symptoms between the two arms limiting the disruptive impact of the trial results.
One of the most interesting findings is the lower number of tests in the Precision strategy besides less futile and more effective catheterization (76 % reduction of invasive coronary angiography without obstructive CAD). This data should implicate important economic and safety issues.
A longer follow-up could determine significant changes in mortality and MI too.
The trial is definitely important for its contribution to the scenario of low-intermediate risk patients defining an effective and advisable approach in stable and suspected CAD.
References
- Newby D, Williams M, Hunter A, Pawade T, Shah A, Flapan A, Forbes J, Hargreaves A, Leslie S, Lewis S, McKillop G, McLean S, Reid J, Spratt J, Uren N, Timmis A, Berry C, Boon N, Clark L, Craig P, Barlow T, Flather M, McCormack C, Roditi G, van Beek E, Shepherd S, Bucukoglu M, Assi V, Parker R, Krishan A, Wee F, Wackett A, Walker A, Milne L, Oatey K, Neary P, Donaldson G, Fairbairn T, Fotheringham M, Hall F, Glen S, Perkins S, Taylor F, Cram L, Beveridge C, Cairns A, Dougherty F, Eteiba H, Rae A, Robb K, Crawford W, Clarkin P, Lennon E, Houston G, Pringle S, Ramkumar PG, Sudarshan T, Fogarty Y, Barrie D, Bissett K, Dawson A, Dundas S, Letham D, O’neill L, Ritchie V, Weir-Mccall J, Dougall H, Ahmed F, Cormack A, Findlay I, Hood S, Murphy C, Peat E, McCabe L, McCubbin M, Allen B, Behan M, Bertram D, Brian D, Cowan A, Cruden N, Denvir M, Dweck M, Flint L, Fyfe S, Grubb N, Keanie C, Lang C, Macgillivray T, Maclachlan D, Macleod M, Mirsadraee S, Morrison A, Mills N, Northridge D, Phillips A, Queripel L, Weir N, Jacob A, Bett F, Divers F, Fairley K, Keegan E, White T, Fowler J, Gemmill J, McGowan J, Henry M, Francis M, Sandeman D, Dinnel L, Bloomfield P, Henriksen P, Macleod D, Mangion K, Mordi I, Tzemos N, Connolly E, Boylan H, Brown A, Farrell L, Frood A, Glover C, Johnstone J, Lanaghan K, McGlynn D, McGregor L, McLennan E, Murdoch L, Paterson V, Teyhan F, Teenan M, Woodward R, Steedman T. CT coronary angiography in patients with suspected angina due to coronary heart disease (SCOT-HEART): An open-label, parallel-group, multicentre trial. Lancet. 2015;385:2383–91.
- Nanna MG, Vemulapalli S, Fordyce CB, Mark DB, Patel MR, Al-Khalidi HR, Kelsey M, Martinez B, Yow E, Mullen S, Stone GW, Ben-Yehuda O, Udelson JE, Rogers C, Douglas PS. The prospective randomized trial of the optimal evaluation of cardiac symptoms and revascularization: Rationale and design of the PRECISE trial. Am Heart J. 2022;245:136–48.
- Schulman-Marcus J, Hartaigh BT, Giambrone AE, Gransar H, Valenti V, Berman DS, Budoff MJ, Achenbach S, Al-Mallah M, Andreini D, Cademartiri F, Callister TQ, Chang HJ, Chinnaiyan K, Chow BJW, Cury R, Delago A, Hadamitzky M, Hausleiter J, Feuchtner G, Kim YJ, Kaufmann PA, Leipsic J, Lin FY, Maffei E, Pontone G, Raff G, Shaw LJ, Villines TC, Dunning A, Min JK. Effects of cardiac medications for patients with obstructive coronary artery disease by coronary computed tomographic angiography: results from the multicenter CONFIRM registry. Atherosclerosis. 2015;238:119–25.
- Gulati M, Levy PD, Mukherjee D, Amsterdam E, Bhatt DL, Birtcher KK, Blankstein R, Boyd J, Bullock-Palmer RP, Conejo T, Diercks DB, Gentile F, Greenwood JP, Hess EP, Hollenberg SM, Jaber WA, Jneid H, Joglar JA, Morrow DA, O’Connor RE, Ross MA, Shaw LJ. 2021 AHA/ACC/ASE/CHEST/SAEM/SCCT/SCMR Guideline for the Evaluation and Diagnosis of Chest Pain: A Report of the American College of Cardiology/American Heart Association Joint Committee on Clinical Practice Guidelines. J Am Coll Cardiol. 2021;78:e187–285.
- Neumann FJ, Sechtem U, Banning AP, Bonaros N, Bueno H, Bugiardini R, Chieffo A, Crea F, Czerny M, Delgado V, Dendale P, Knuuti J, Wijns W, Flachskampf FA, Gohlke H, Grove EL, James S, Katritsis D, Landmesser U, Lettino M, Matter CM, Nathoe H, Niessner A, Patrono C, Petronio AS, Pettersen SE, Piccolo R, Piepoli MF, Popescu BA, Räber L, Richter DJ, Roffi M, Roithinger FX, Shlyakhto E, Sibbing D, Silber S, Simpson IA, Sousa-Uva M, Vardas P, Witkowski A, Zamorano JL, Achenbach S, Agewall S, Barbato E, Bax JJ, Capodanno D, Cuisset T, Deaton C, Dickstein K, Edvardsen T, Escaned J, Funck-Brentano C, Gersh BJ, Gilard M, Hasdai D, Hatala R, Mahfoud F, Masip J, Muneretto C, Prescott E, Saraste A, Storey RF, Svitil P, Valgimigli M, Aboyans V, Baigent C, Collet JP, Dean V, Fitzsimons D, Gale CP, Grobbee DE, Halvorsen S, Hindricks G, Iung B, Jüni P, Katus HA, Leclercq C, Lewis BS, Merkely B, Mueller C, Petersen S, Touyz RM, Benkhedda S, Metzler B, Sujayeva V, Cosyns B, Kusljugic Z, Velchev V, Panayi G, Kala P, Haahr-Pedersen SA, Kabil H, Ainla T, Kaukonen T, Cayla G, Pagava Z, Woehrle J, Kanakakis J, Toth K, Gudnason T, Peace A, Aronson D, Riccio C, Elezi S, Mirrakhimov E, Hansone S, Sarkis A, Babarskiene R, Beissel J, Cassar Maempel AJ, Revenco V, de Grooth GJ, Pejkov H, Juliebø V, Lipiec P, Santos J, Chioncel O, Duplyakov D, Bertelli L, Dikic AD, Studencan M, Bunc M, Alfonso F, Back M, Zellweger M, Addad F, Yildirir A, Sirenko Y, Clapp B. 2019 ESC Guidelines for the diagnosis and management of chronic coronary syndromesThe Task Force for the diagnosis and management of chronic coronary syndromes of the European Society of Cardiology (ESC). Eur Heart J. 2020;41:407–77.





No comments yet!