01 Sep 2025
The AQUATIC trial: Assessment of quitting versus using aspirin therapy in patients with stabilized coronary artery disease after stenting who require long-term oral anticoagulation
Reported from ESC Congress 2025
Chiara De Biase provides her take on the results of AQUATIC presented by Martine Gilard at ESC Congress 2025 in Madrid.
AQUATIC is an investigator-driven, randomized, double-blind, placebo-controlled, multicenter trial aiming to compare the use of long-term aspirin versus placebo in patients with chronic coronary syndrome (CCS) and stenting who require long-term oral anticoagulation (OAC). The trial was presented in a Hot Line session at the latest ESC Congress 2025 and simultaneously published in the New England Journal of Medicine.1
Why this study – the rationale/objective?
The optimal antithrombotic management for patients requiring long-term OAC after stenting remains a matter of debate.
Previous studies reported that in patients with the need for OAC therapy, the addition of antiplatelet therapy increased bleeding without a clear benefit on ischemic outcomes.
However, these studies presented several biases, including an open-label design, patients without stenting, and Asian patients with different atherothrombotic and bleeding risks.
Thus, the rationale behind the AQUATIC trial comes from the need to better treat specific patients with CCS and stenting, who require long-term OAC, in order to optimize the thrombotic and ischemic risk in this population.
After stent implantation, many patients with CCS are at high risk for future cardiovascular events due to risk factors like diabetes, chronic kidney disease (CKD) and diffuse multivessel disease (MVD). Anyway, some of them require long-term anticoagulation, particularly due to atrial fibrillation (AF). Managing the risk of further cardiovascular events in these patients is challenging, and there is limited trial evidence to guide the optimal antithrombotic strategy, considering the higher bleeding risk related to OAC treatment. To better understand the management of these patients, the AQUATIC trial analyzes the efficacy and safety of adding aspirin to OAC, a combination that is commonly used for this high-risk population in clinical practice.
How was it executed - the methodology
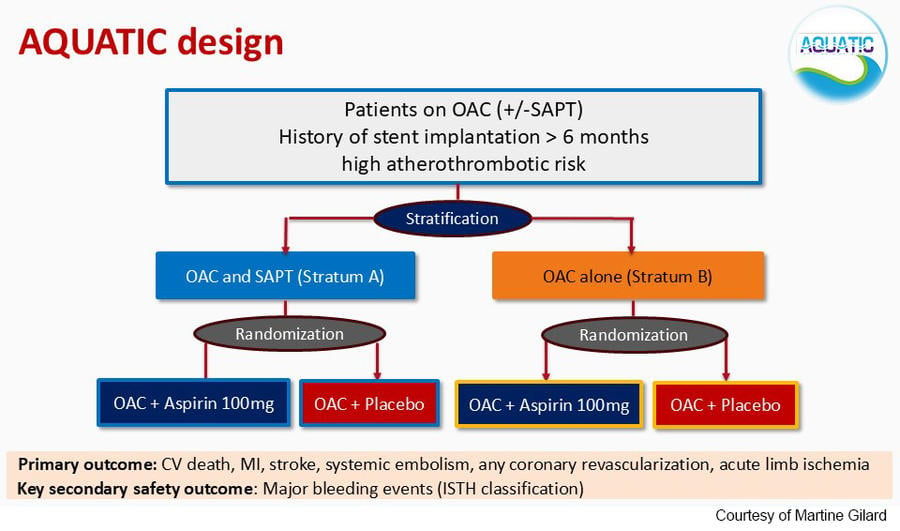
The AQUATIC is a double-blind, placebo-controlled, parallel-group, randomized trial conducted at 51 centers in France.
Eligible patients had CCS and stent implantation (>6 months before), were at high atherothrombotic risk and required long-term OAC for any reason (mainly AF).
High atherothrombotic risk was defined as either a history of percutaneous coronary intervention (PCI) during an acute coronary syndrome (ACS) (with ≥1 stent(s) >6 months) or history of PCI (>6 months) outside the context of ACS but with high-risk features such as diabetes, CKD, diffuse MVD (involvement of 3 coronary vessels), history of complex PCI (left main, chronic total occlusion, 3 lesions), stent thrombosis or peripheral artery disease (PAD).
Main exclusion criteria included recent coronary or bleeding event, haemorrhagic disease, stroke within 1 month, any history of haemorrhagic stroke, contraindication to aspirin or OAC, severe renal or hepatic insufficiency, severe uncontrolled heart failure.
The final intention to treat population included 872 patients.
Concerning the baseline patient characteristics, they were very well balanced between the 2 arms. Mean age was 71 years, with 85% of males, 70% of the PCI was performed during an acute coronary syndrome, and a quarter of the patients underwent PCI between 6 months and 1 year at randomization. The main indication of oral anticoagulation was AF in over 87% of cases, with a large majority of OACs.
Patients were randomised 1:1 to aspirin or placebo on top of OAC (either a direct OAC or vitamin K antagonists). Permuted block randomization was stratified by centre, type of oral anticoagulant (OAC or vitamin K antagonist-VKA), and baseline antithrombotic regimen at inclusion (stratum A or stratum B, Figure).
Complete follow-up was obtained for 310 patients in the OAC + aspirin group and 319 in the placebo group.
What is the main result?
The primary efficacy endpoint was a composite of cardiovascular death, myocardial infarction, stroke, systemic embolism, coronary revascularization and acute limb ischemia.
The primary efficacy outcome occurred in significantly more patients in the aspirin group than the placebo group (16.9% vs. 12.1%; adjusted hazard ratio [HR] 1.53; 95% confidence interval [CI] 1.07 to 2.18; p=0.019). All-cause death also occurred in significantly more patients with aspirin vs. placebo (13.4% vs. 8.4%; adjusted HR 1.72; 95% CI 1.14 to 2.58; p=0.010).
The key secondary safety endpoint was major bleeding according to the International Society on Thrombosis and Hemostasis (ISTH) definition.
The risk of major bleeding was more than three-fold higher in the aspirin group than the placebo group (10.2% vs. 3.4%; HR 3.35; 95% CI 1.87 to 6.00; p<0.0001).
According to these results, the trial was stopped early on the advice of the independent Data Safety Monitoring Board after a median follow-up of 2.2 years due to an excess of all-cause mortality in the aspirin group. A total of 467 and 395 serious adverse events were reported in the aspirin and placebo groups, respectively.
In conclusion, the AQUATIC trial reported an increased rate of major cardiovascular events, all-cause mortality and major bleeding in the aspirin group of patients with CCS at high atherothrombotic risk requiring long-term OAC therapy. Thus, aspirin negatively impacted the outcomes at a median follow-up of 2.2 years, and according to these results, its use should be discouraged.
Other studies have investigated antithrombotic therapy for stable coronary artery disease and AF, 2,3 but this is the first randomized trial to include patients who had prior stenting and a high atherothrombotic risk.
Critical reading and the relevance for clinical practice
The balance between the haemorrhagic and ischemic risk in patients with previous stenting and the need for long-term OAC is still an open debate in current clinical practice.
To optimize this balance in this population is crucial, considering that the need for OAC is often driven by AF, and that approximately one in five patients with AF need to undergo PCI with stenting.
These patients need stroke prevention on one side, justifying the indication to anticoagulation (with OACs preferred over the VKA), and stent thrombosis and MI prevention on the other, leading to indication to dual antiplatelet therapy (DAPT).
If 1+2 is correct, a triple antithrombotic therapy is finally required in this population. But how long and how strong should this double-triple therapy be in order to result in effective and protective in these patients?
It's well described that the combination of an OAC plus DAPT leads to an increased bleeding risk and major bleeding, leading to earlier mortality.
Five randomized-controlled trials have shown that double compared with triple antithrombotic therapy reduced major or clinically relevant non-major bleeding, without a significant increase of ischemic events, considering as recommended the use of double antithrombotic therapy (OAC plus P2Y12 receptor inhibitor, mostly clopidogrel) after a 1–4 week period of triple antithrombotic therapy in CCS patients with AF undergoing PCI.
More in detail, the AUGUSTUS trial (Open-Label, 2 × 2 Factorial, Randomized Controlled, Clinical Trial to Evaluate the Safety of Apixaban versus Vitamin K Antagonist and Aspirin versus Aspirin Placebo in Patients with AF and Acute Coronary Syndrome or Percutaneous Coronary Intervention),4 additionally demonstrated that the apixaban reduced major or clinically relevant non-major bleeding compared with VKA, independently of a double or triple antithrombotic regimen.
This trial, together with additional meta-analyses, demonstrated also that aspirin compared with placebo reduced stent thrombosis events, which occurred mainly during the first 30 days after PCI, while increasing bleeding risk. 5-7
According to this evidence, the latest 2024 ESC Guidelines for CCS recommended a double antithrombotic therapy with OAC and clopidogrel for up to 12 months for CCS patients with AF undergoing PCI,8 with additional aspirin only for a limited initial period (from during PCI up to a maximum of 30 days in patients at high ischemic risk). What’s more, in patients with the highest bleeding risk, clopidogrel discontinuation at 6 (or even 3) months post-PCI and continuation of OAC alone may be considered when ischemic risk is not high (Class IIb/C).
This recommendation is translated into a common clinical practice of great discrepancy of treatment between patients, without a certain timing for continuing or interrupting the aspirin/clopidogrel treatment together with OACs.
In patients at high thrombotic risk, it seems reasonable to continue a triple therapy for 1 month in order to reduce the risk of stent thrombosis in the first 30 days from PCI, but what about long-term management?
The AQUATIC tried to solve the doubt of quitting vs. using aspirin for longer than 6 months after stenting in patients requiring OAC. The additional value of this trial is the strong design and the robustness of the results. According to this randomized, double-blind, multicentre trial, the use of aspirin is discouraged after 6 months from PCI in patients at high thrombotic risk because of an excess of all-cause mortality and adverse events in the aspirin group.
What is considerable is that the event rates were around seven times higher in AQUATIC than in previous trials.
Thus, the AQUATIC trial seems to solve the doubt of treatment management in CCS patients with stenting and need for OAC, and it could probably impact next ESC Guidelines according to its strong randomized design and results showing the risk of prolonging aspirin use in this specific population.
References
- ‘The AQUATIC trial’ presented during HOT LINE 6 on 31 August 2025 at 08:51 to 09:01 in Madrid (Main Auditorium) and simultaneously published in the New England Journal of Medicine.
- Yasuda S, Kaikita K, Akao M, et al. Antithrombotic therapy for atrial fibrillation with stable coronary disease. N Engl J Med. 2019;381:1103–1113.
- Cho MS, Kang DY, Ahn JM, et al. Edoxaban antithrombotic therapy for atrial fibrillation and stable coronary artery disease. N Engl J Med. 2024;391:2075–2086.
- Lopes RD, Heizer G, Aronson R, Vora AN, Massaro T, Mehran R, et al. Antithrombotic therapy after acute coronary syndrome or PCI in atrial fibrillation. N Engl J Med 2019; 380:1509–24.
- Galli M, Andreotti F, Porto I, Crea F. Intracranial haemorrhages vs. stent thromboses with direct oral anticoagulant plus single antiplatelet agent or triple antithrombotic therapy: a meta-analysis of randomized trials in atrial fibrillation and percutaneous coronary intervention/acute coronary syndrome patients. Europace 2020;22:538–46.
- Gargiulo G, Goette A, Tijssen J, Eckardt L, Lewalter T, Vranckx P, et al. Safety and efficacy outcomes of double vs. triple antithrombotic therapy in patients with atrial fibrillation following percutaneous coronary intervention: a systematic review and meta-analysis of non-vitamin K antagonist oral anticoagulant-based randomized clinical trials. Eur Heart J 2019;40:3757–67.
- Alexander JH, Wojdyla D, Vora AN, Thomas L, Granger CB, Goodman SG, et al. Risk/ benefit tradeoff of antithrombotic therapy in patients with atrial fibrillation early and late after an acute coronary syndrome or percutaneous coronary intervention: insights from AUGUSTUS. Circulation 2020;141:1618–27.
- Vrints C, Andreotti F, Koskinas KC, et al. 2024 ESC Guidelines for the management of chronic coronary syndromes. Eur Heart J. 2024;45:3415–3537.




2 comments
thanks we are supporting monotherapy after 1 year of stent implantation this study also support the ADAPT-AF-DES trail in which aspirin is replaced with clopidogrel in such senerio monotherapy is the best sometime it is necessary to decide on the behaf of patient vessel complexity thank you
thanks guidlines supporting monotherapy after 1 year of stent implantation this study also support the ADAPT-AF-DES trail in which aspirin is replaced with clopidogrel in such senerio monotherapy is the best sometime it is necessary to decide on the behaf of patient vessel complexity thank you