ECMO-CS – Extracorporeal membrane oxygenation in the therapy of cardiogenic shock: primary results from the multicenter, randomized ECMO-CS trial
Reported from AHA 2022
Mirvat Alasnag provides her take on the ECMO-CS trial, which was presented during AHA 2022 in Chicago, and simultaneously published in Circulation.
Why this study – the rationale/objective?
For patients in cardiogenic shock, the 2021 European Society of Cardiology Guidelines currently recommend oxygen administration, affording it a class I recommendation, inotropic & vasopressor support assigned a class IIb recommendation, and ventilation and mechanical circulatory support (MCS), which are assigned a class IIa recommendation1. Despite all exhaustive measures to reduce mortalities in those with cardiogenic shock, the rate remains disturbingly high (40-60 %), even with advanced therapies including MCS2.
It is often speculated that intensive measures, such as the use of MCS, are initiated too late. As such, the hypothesis of the ECMO-CS (Extracorporeal Membrane Oxygenation in the Therapy of Cardiogenic Shock) trial was that immediate implementation of veno-arterial (VA)-ECMO in patients with rapidly deteriorating or severe cardiogenic shock would result in a better hospital survival.
How was it executed - the methodology?
The design for this study was previously published3. Briefly, this was a multicenter, randomized, investigator-initiated study. The trial design was a 1:1 randomization of patients to either immediate VA-ECMO or an initial conservative strategy, whereby VA-ECMO could be initiated downstream in case of worsening hemodynamic status. A rapidly deteriorating hemodynamic status or severe cardiogenic shock was defined as:
- SCAI (Society of Cardiovascular Angiography & Interventions) stage D or E with repeated vasopressor to maintain a mean arterial pressure (MAP) > 50 mmHg.
- SCAI stage D with hemodynamics suggestive of severe shock ie Cardiac Index (CI) < 2.2 L/min/m2 + norepinephrine + dobutamine OR systolic blood pressure < 100 mmHg + norepinephrine + dobutamine + ejection fraction, 35 % or 35-55 % with severe mitral regurgitation or aortic stenosis.
- Lactate > 3 mmol/L and SVO2 < 50 % suggesting tissue hypoperfusion.
Hypovolemia was an exclusion defined by a central venous pressure > 7 mmHg or pulmonary artery wedge pressure > 12 mmHg.
The primary endpoint was the composite of death from any cause, resuscitated circulatory arrest, and implementation of another mechanical circulatory support device at 30 days. With a sample size of 120, the study had 80 % power to detect 50 % reduction of the primary endpoint.
What is the main result?
A total of 117 patients were included in the final analysis. Immediate VA-ECMO was instituted in 58, and 59 were assigned to the initial conservative arm. The median age was 67 years with a predominantly male population (74 %). The presentation was ST-elevation myocardial infarction (STEMI) in 51 % with the remaining having a Non-STEMI, decompensated heart failure, or a mechanical complication of myocardial infarction.
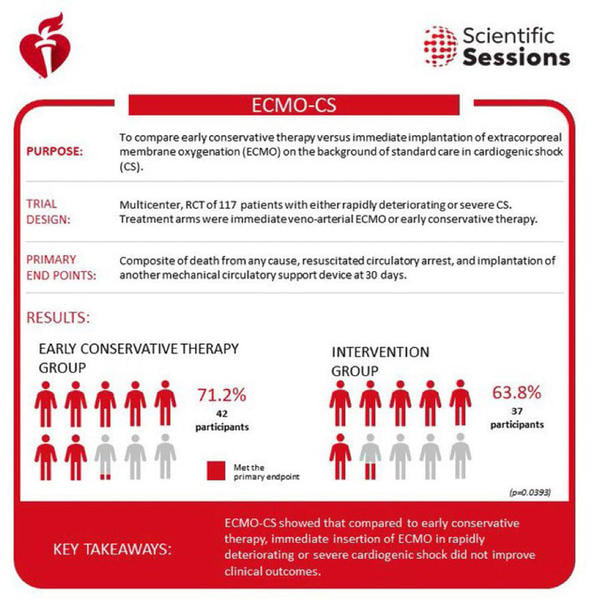
Lactate levels were higher in the immediate ECMO arm (5.3 versus 4.7 mmol/L). Mechanical ventilation was necessary for 74 % of the total population and norepinephrine was the most commonly used inotropic agent (86 %). The results were rather disappointing. The composite primary endpoint occurred in 63.8 % of the immediate VA-ECMO group, and 71.2 % of the initial conservative group, with a hazard ratio (HR) 0.72 ; 95 % confidence intervals [CI], 0.46 to 1.12; P = 0.21. In the initial conservative group, 39 % eventually required VA-ECMO. There was no statistical difference in the 30-day rates of the other endpoints:
Endpoint | VA-ECMO | Initial Conservative Group |
Resuscitated cardiac arrest | 10.3 % | 13.6 % |
All-cause mortality | 50.0 % | 47.5 % |
Serious adverse events | 60.3 % | 61.0 % |
Serious adverse events, although numerically higher in the immediate VA-ECMO group, did not reach statistical significance:
Adverse Event | VA-ECMO Group | Initial Conservative Group |
Sepsis | 39.7 % | 39.0 % |
Pneumonia | 31.0 % | 30.5 % |
Stroke | 5.2 % | 0.0 % |
Leg ischemia | 13.8 % | 5.1 % |
Bleeding | 31.0 % | 20.3 % |
Critical reading and the relevance for clinical practice
There was no notable difference amongst the sub-groups (albeit the numbers were too small for a meaningful analysis); for example, by etiology of shock or whether this was a bridge to more definitive support. Of note, only 3 received ECMO as a bridge to a long-term support device such as Heartmate and 2 to short-term devices. None of the patients were bridged to transplant. These results are aligned with previously published data examining circulatory support in cardiogenic shock with fewer fortuitous devices, namely, the intra-aortic balloon pump. In the IABP-Shock II trial (Intra-Aortic Balloon Pump in Cardiogenic Shock) the 30-day all-cause mortality was 39.7 % versus 41.3 %; P=0.694. We await other randomized trials in cardiogenic shock with other commonly used devices such as the Impella CP in addition to ECMO and IABP5-7.
What we’ve learned from the ECMO-CS trial is that immediate implementation of VA-ECMO in patients with rapidly deteriorating or severe cardiogenic shock (SCAI stage D-E) is feasible. This was an extremely high-risk population with a 50 % 30-day mortality rate. The reported adverse events suggest the use of ECMO in such a high-risk population is safe. However, the clinical outcomes are comparable to an early conservative strategy; although 39 % of the early conservative group eventually required ECMO support due to continued hemodynamic deterioration.
The trial was conducted exclusively in the Czech Republic, making it difficult to generalize the results to other healthcare systems and populations. The small sample size in each group precluded investigators from identifying smaller rates of risk reduction. VA-ECMO was a bailout strategy in the early conservative group. The definition of worsening shock remains vague and largely dependent on worsening lactate levels. The rate of lactate increase was not a rigid parameter. Since the timing of downstream ECMO use and unloading of the left ventricle were not reported, the technical adequacy of the circulatory support remains less understood.
The benefits of ECMO in terms of improving end-organ perfusion are well established.8 Whether that translates into a survival benefit is yet to be determined. This trial suggests that there is no penalty in early ECMO and no survival benefit either. We are still at a loss for standardized definitions for escalation of therapy which cannot be clarified by the ECMO-CS trial.
ECMO-CS - Key Takeaways - Source: AHA
References
- Collet JP, Thiele H, Barbato E, Barthélémy O, Bauersachs J, Bhatt DL, Dendale P, Dorobantu M, Edvardsen T, Folliguet T, Gale CP, Gilard M, Jobs A, Jüni P, Lambrinou E, Lewis BS, Mehilli J, Meliga E, Merkely B, Mueller C, Roffi M, Rutten FH, Sibbing D, Siontis GCM; ESC Scientific Document Group. 2020 ESC Guidelines for the management of acute coronary syndromes in patients presenting without persistent ST-segment elevation. Eur Heart J. 2021 Apr 7;42(14):1289-1367. Erratum in: Eur Heart J. 2021 May 13.
- Shaefi S, O'Gara B, Kociol RD, Joynt K, Mueller A, Nizamuddin J, Mahmood E, Talmor D, Shahul S. Effect of cardiogenic shock hospital volume on mortality in patients with cardiogenic shock. J Am Heart Assoc. 2015 Jan 5;4(1):e001462.
- Ostadal P, Rokyta R, Kruger A, Vondrakova D, Janotka M, Smíd O, Smalcova J, Hromadka M, Linhart A, Bělohlávek J. Extra corporeal membrane oxygenation in the therapy of cardiogenic shock (ECMO-CS): rationale and design of the multicenter randomized trial. Eur J Heart Fail. 2017 May;19 Suppl 2:124-127.
- Thiele H, Zeymer U, Neumann FJ, Ferenc M, Olbrich HG, Hausleiter J, Richardt G, Hennersdorf M, Empen K, Fuernau G, Desch S, Eitel I, Hambrecht R, Fuhrmann J, Bohm M, Ebelt H, Schneider S, Schuler G and Werdan K. Intraaortic balloon support for myocardial infarction with cardiogenic shock. N Engl J Med. 2012;367:1287-96.
- Tsangaris A, Alexy T, Kalra R, Kosmopoulos M, Elliott A, Bartos JA and Yannopoulos D. Overview of Veno-Arterial Extracorporeal Membrane Oxygenation (VA-ECMO) Support for the Management of Cardiogenic Shock. Front Cardiovasc Med. 2021;8:686558.
- Udesen NJ, Moller JE, Lindholm MG, Eiskjaer H, Schafer A, Werner N, Holmvang L, Terkelsen CJ, Jensen LO, Junker A, Schmidt H, Wachtell K, Thiele H, Engstrom T, Hassager C and DanGer Shock i. Rationale and design of DanGer shock: Danish-German cardiogenic shock trial. American heart journal. 2019;214:60-68.
- Banning AS, Adriaenssens T, Berry C, Bogaerts K, Erglis A, Distelmaier K, Guagliumi G, Haine S, Kastrati A, Massberg S, Orban M, Myrmel T, Vuylsteke A, Alfonso F, Van de Werf F, Verheugt F, Flather M, Sabate M, Vrints C, Gershlick AH and Collaborators. Veno-arterial extracorporeal membrane oxygenation (ECMO) in patients with cardiogenic shock: rationale and design of the randomised, multicentre, open-label EURO SHOCK trial. EuroIntervention. 2021;16:e1227-e1236.
- Lawler PR, Silver DA, Scirica BM, Couper GS, Weinhouse GL, Camp PC Jr. Extracorporeal membrane oxygenation in adults with cardiogenic shock. Circulation. 2015 Feb 17;131(7):676-80.




No comments yet!