TRILUMINATE pivotal: A landmark randomized clinical trial of transcatheter tricuspid valve edge-to-edge repair for tricuspid regurgitation
Reported from ACC.23/WCC
Jonathan Curio and Alex Sticchi provide their take on this clinical trial which was presented by Paul Sorajja at the American College of Cardiology Scientific Session (ACC.23/WCC).

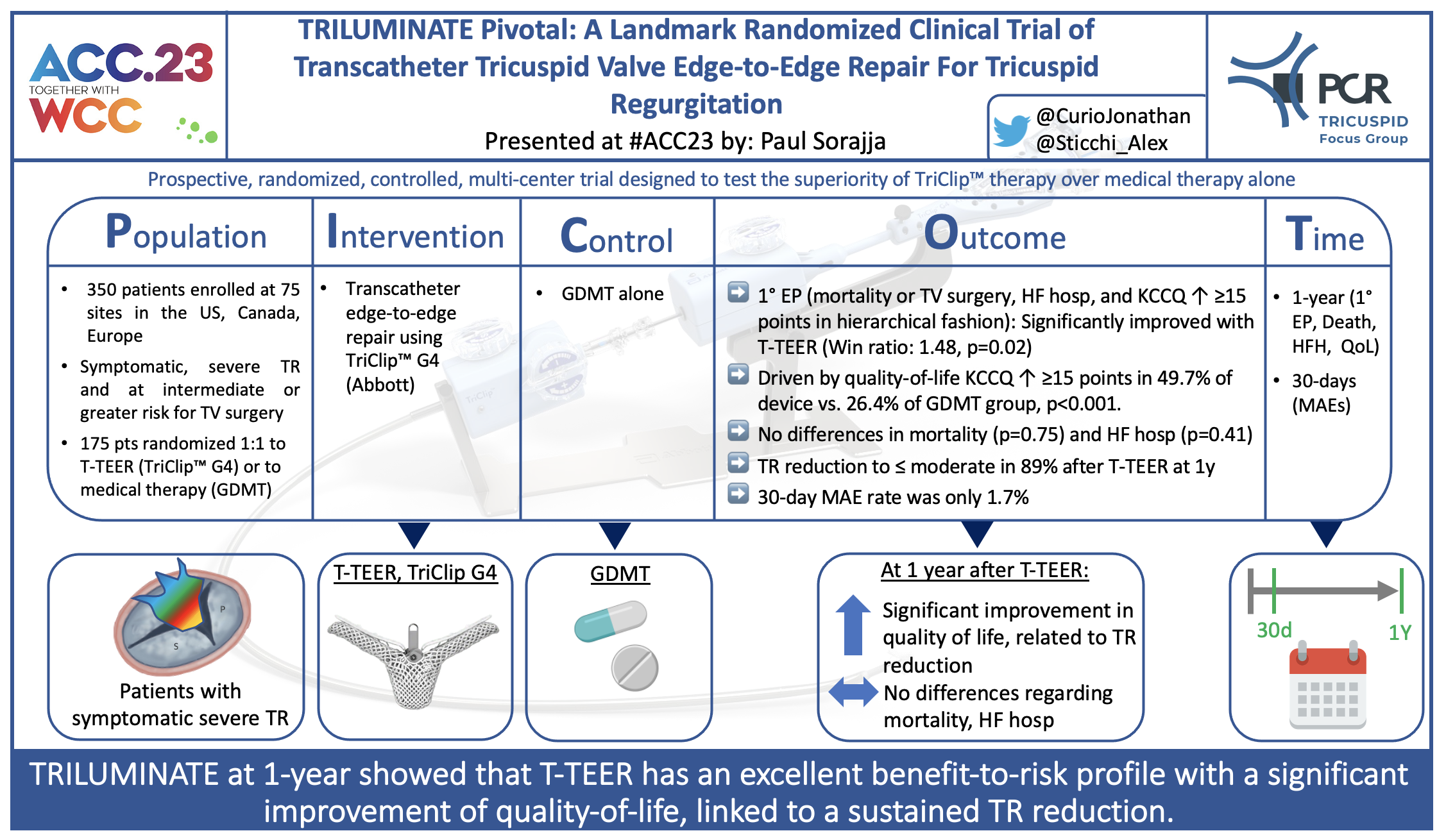
Picot scheme highlighting key aspects of the study. Courtesy of @CurioJonathan and @Sticchi_Alex. Source: PCRonline.com
Why this study? – the rationale/objective
Symptomatic severe tricuspid regurgitation (TR) has been identified as an independent predictor of increased mortality(1). However, given high peri-procedural mortality of isolated surgical treatment, several devices for less invasive transcatheter tricuspid valve intervention have been developed(2). The largest experience, so far, has been gathered for tricuspid transcatheter edge-to-edge repair (T-TEER).
Data from the initial prospective, unblinded, single-arm TRILUMINATE study (NCT03227757) and data from the TriValve registry showed safety and efficacy of T-TEER(3,4). However, so far, no randomized data comparing T-TEER with optimized medical therapy alone was available.
The TRILUMINATE Pivotal trial, randomizing T-TEER against guideline-directed medical therapy, represents the first randomized data available in the field of transcatheter tricuspid valve interventions.
How was it executed? – the methodology
The TRILUMINATE Pivotal trial is a multicenter randomized controlled trial that 1:1 randomized patients to either T-TEER with the TriClip device (Abbott Medical) or medical therapy alone. All patients suffered from symptomatic significant tricuspid regurgitation (severe/massive/torrential: 97.7% in device arm, 98.8% in control arm) and were at intermediate or greater risk for mortality or morbidity with tricuspid valve surgery.
- Primary endpoint: Hierarchical composite of all-cause mortality or tricuspid valve (TV) surgery, heart failure hospitalizations, and assessment of quality-of-life improvement using the KCCQ (at 12 months)
- Secondary endpoints: freedom from MAE after procedure (at 30 days), change in TR from severe to ≤ moderate (at 30-days), change in QoL by KCCQ and 6-minute-walk-test (at 12 months).
What is the main result?
The study randomized more than 450 patients suffering from symptomatic severe TR enrolled at 75 sites in the US, Canada, and Europe. 12-month follow-up is reached by 350 patients (175 with T-TEER or medical therapy, each).
Mean age was 78 years, 56% (device) and 54% (control) were female, and NYHA class III/IV was reported in 59.4% (device) and 55.4% (control) at baseline.
- For the primary endpoint at 12 months, T-TEER with TriClip was superior to medical therapy alone, driven by a significant improvement in quality-of-life by KCCQ (tested in a hierarchical fashion using Finkelstein-Schoenfeld methodology: Win ratio, 1.48 [1.06 – 2.13], p=0.02)
- At 12 months, freedom from all-cause death or TV Surgery (90.6% in device vs. 89.4% in control group) and freedom from heart failure hospitalizations (85.1% in device vs. 87.9% in control group) were without significant differences between groups
- Quality-of-life at 12 months was significantly better in the T-TEER group, where it improved by ≥ 15 KCCQ points in 49.7% patients compared to only 26.4% in the medical group; degree of improvement in quality of life was directly related to degree of TR reduction
- At 30 days, freedom from MAE in the device group was 98.3%, and TR grade was ≤ moderate in 87.0% (vs. 4.8% in control group, p>0.001).
Critical reading and the relevance for clinical practice
First and foremost, it is important to highlight that this trial means a historical landmark in the field of transcatheter tricuspid valve interventions, as it represents the first randomized data for such therapy in the population of severe TR patients.
The superiority of T-TEER according to the combined primary endpoint at 12 months was notably driven by quality-of-life improvements, which were linked to the degree of TR reduction. Hard endpoints, however, that are less prone to bias in unblinded patients so far did not show any differences between device and control group. This also raises important questions for longer-term follow-up, as for example in mitral TEER mortality benefits are seen from around 9 months after the procedure onwards.
Given the link between TR reduction and improvement in quality-of-life, it is important to highlight that T-TEER, even though it significantly reduces TR, does not entirely eliminate TR. In the TRILUMINATE pivotal trial, 38% of patients showed moderate and 11% showed severe TR at 12 months. Still, it is encouraging to see that such TR reduction means a marked improvement compared to the very initial experience with TriClip in the single-arm TRILUMINATE study (n=85 patients), where at 1 year 29% showed severe/massive/torrential TR and only 37% showed none/trace TR (vs. 51% now in this TRILUMINATE RCT with the latest generation TriClip G4 available in around half of the trial)(3). Thus, operator/center experience as well as patient selection likely are fundamental for procedural success, and finally, linked to that, for longer-term outcomes. These factors might further improve with growing experience in patient selection, procedural guidance, device iterations and execution of the device implantation itself. Furthermore, based on these findings, other transcatheter tricuspid valve interventions such as orthotopic valve replacement, fully abolishing TR, may have a more pronounced impact on clinical outcomes.
As in patients such as those enrolled in the trial TR significantly impacts quality-of-life as well as survival and as surgical mortality remains high, these patients were lacking a proper treatment and until now represented an unmet clinical need. Given the high safety of T-TEER, as confirmed by this trial with a rate of only 1.7% of MAE, with this form of transcatheter tricuspid valve intervention for these formerly untreated patients, a meaningful improvement in quality-of-life at a very reasonable risk-to-benefit ratio is achievable. However, patients currently undergoing T-TEER still rather are later-stage heart failure patients, questioning the appropriate timing of TR intervention and whether treatment effects might be different in other TR cohorts.
Longer-term follow-up of this trial as well as other randomized trials of T-TEER, namely the TRI-FR study (NCT04646811) and the Clasp II TR trial (NCT04097145), as well as an RCT comparing tricuspid valve replacement with optimal medical therapy (TRISCEND II, NCT04482062) that currently all are ongoing, will add further data to the field and will inform more precisely on ideal selection of patients and how much these patients may benefit from transcatheter tricuspid valve intervention.
Finally, this first randomized data in the field of transcatheter tricuspid valve interventions gives us the important message, that at 1 year T-TEER with TriClip is an effective and very safe procedure, significantly improving quality-of-life, linked to the achieved TR reduction. Longer-term data will inform on the durability of these results and further analyses may inform precisely which TR patients will benefit most from intervention.
The PCR Tricuspid Focus Group will hold a dedicated Webinar on April 4th (5:00 pm, CET), discussing the impact of the TRILUMINATE trial results on clinical practice and giving some additional perspectives on how to interpret them – if interested, easily register here:
References
- Nath J, Foster E, Heidenreich PA. Impact of tricuspid regurgitation on long-term survival. J Am Coll Cardiol 2004;43:405-9.
- Praz F, Muraru D, Kreidel F et al. Transcatheter treatment for tricuspid valve disease. EuroIntervention 2021;17:791-808.
- Lurz P, Stephan von Bardeleben R, Weber M et al. Transcatheter Edge-to-Edge Repair for Treatment of Tricuspid Regurgitation. J Am Coll Cardiol 2021;77:229-239.
- Mehr M, Taramasso M, Besler C et al. 1-Year Outcomes After Edge-to-Edge Valve Repair for Symptomatic Tricuspid Regurgitation: Results From the TriValve Registry. JACC Cardiovasc Interv 2019;12:1451-1461.
Authors





No comments yet!