26 Aug 2023
P2Y12 inhibitor monotherapy vs DAPT after PCI with BP-EES: The SHARE Randomized Clinical Trial
Reported from ESC Congress 2023
Elad Asher provides his take on the final results of the SHARE (SHort-term Dual Antiplatelet Therapy After Deployment of BioabsoRbable Polymer Everolimus-Eluting Stent) trial, which were presented during the ESC 2023 congress in Amsterdam by Pil-Ki Min.
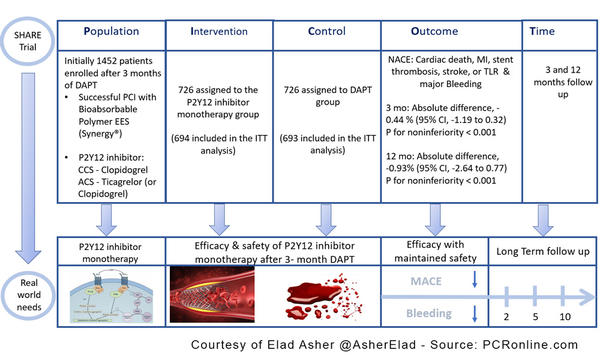
PICOT analysis of Share Trial - Courtesy of Elad Asher @AsherElad - Source: PCRonline.com
Why this study – the rationale/objective?
- Recommendations for the use of dual antiplatelet therapy (DAPT) after percutaneous coronary intervention (PCI) have changed with advances in drug-eluting stents (DES) technology.
- The aim of the current trial was to compare the efficacy & safety of P2Y12 inhibitor monotherapy after 3- 3-month DAPT vs. 12-month DAPT in patients who underwent successful PCI with the latest DES.
How was it executed – the methodology?
- A multicenter (20 hospitals in South Korea), prospective, randomized, open-label, non-inferiority trial
- Enrollment: 2017.12 ~ 2020.12 with follow-up completion: 2022.1.
- SYNERGY stent
- P2Y12 inhibitor: Clopidogrel in CCS
Ticagrelor (or Clopidogrel) in ACS - 1:1 randomization for P2Y12 inhibitor monotherapy vs. DAPT after successful PCI and 3 months of DAPT
Exclusion criteria: Age ≥ 86 years; hemodynamically unstable patients; patients at high risk of bleeding, anemia, or thrombocytopenia; patients requiring oral anticoagulation; life expectancy less than 1 year; patients with a history of intracranial hemorrhage; patients unable to take aspirin, clopidogrel, or ticagrelor due to contraindications of each agent; history of coronary stenting within 1 year; LM disease requiring intervention; CTO lesion requiring intervention; ISR lesion requiring intervention; bifurcation lesions requiring stenting in the side branches; lesions requiring overlap of 3 or more stents.
Primary outcomes:
- Net adverse clinical event (NACE): a composite of MACCE (cardiac death, MI, stent thrombosis, stroke, or TLR) & major bleeding (BARC type 3 or 5 bleeding) (3 ~ 12 mo after the index PCI)
Secondary outcomes:
- MACCE, major bleeding, cardiac death, MI, stent thrombosis, stroke, TLR, TVR, and all-cause death
Major findings
- NACE absolute difference for 3 months, -0.44 % (95% CI, -1.19 to 0.32) P for noninferiority < 0.001
- NACE absolute difference for 12 months, -0.93% (95% CI, -2.64 to 0.77) P for noninferiority < 0.001
- Major bleeding 1 (0.2%) in the monotherapy vs. 5 (0.8%) in DAPT absolute difference -0.6 (-1.33, 0.12) p=0.104
- Stent thrombosis 1 (0.2%) in the monotherapy vs. 1 (0.2%) in DAPT absolute difference 0.03 (-0.55, 0.6) p=0.929
- In subgroups analysis no differences in all sub-groups
Critical reading and the relevance for clinical practice
The results of this study show that in CAD patients undergoing PCI with the latest generation DES, P2Y12 inhibitor monotherapy after 3-month DAPT was not inferior to 12-month DAPT in terms of NACE.
The authors concluded that considering the study population and lower-than-expected event rates, further research is required in other populations.
Should common practice and guidelines be changed?
The SHARE study has several limitations: It was an open-label trial, had low adherence rate to the study drug, lower-than-expected event rate and wide noninferiority margin. Very importantly, the randomization took place only 3 months after the index PCI and after DAPT and lastly, it was performed in South Korea only.
Several randomized control trials and meta-analyses have compared standard 12-month DAPT with ≤6 months DAPT followed by aspirin or P2Y12 inhibitor monotherapy in ACS patients (i.e. TWILIGHT, TICO, STOPDAPT-2-ACS, MASTER DAPT etc.). In accordance with these trials the recent 2023 ACS guidelines state that “Considering the totality of evidence from the scientific literature, alternatives to the default strategy of 12 months DAPT in patients with ACS include shortening the DAPT duration to 1 or 3–6 months (depending on the balance of bleeding and ischemic risks) and de-escalating DAPT from prasugrel/ticagrelor-based DAPT to clopidogrel-based DAPT.
However, it should be noted that much of the evidence on these strategies in ACS patients is derived from trials powered primarily for bleeding outcomes, many of which had a non-inferiority design and were, therefore, not powered to detect potentially relevant differences in ischemic outcomes”.
Moreover, the guidelines add: “These important limitations explain why these strategies should at present remain considered as alternative strategies to the default of 12 months DAPT. From a practical perspective, this means that these strategies should not be employed as a default strategy in the wider ACS population but can be considered when there is a specific motivation for their use (i.e. aiming to reduce the risk of bleeding events in high bleeding risk patients or if there are other specific concerns regarding a 12-month potent P2Y12 inhibitor-based DAPT regimen).
What do you think would be the future of P2Y12 inhibitor monotherapy in patients with ACS?
Latest news from ESC Congress 2023






1 comment
It seems very unlikely that the results of this study can be incorporated into the guideline. Patients' comorbidities can vary internationally. Factors such as weight, dietary habits, sedentary lifestyle, or sports habits can differ from country to country, which will also alter the atherosclerotic process and the rate of in-stent restenosis. Additionally, many centers still do not use next-generation stents and continue to use bare-metal stents. Unfortunately, monotherapy does not appear to be feasible in these patient groups in today's conditions.