25 Oct 2025
STRIVE: intracoronary low-dose recombinant tissue plasminogen activator in primary PCI for STEMI and large thrombus burden
Reported from TCT 2025
Elad Asher provides his take on the STRIVE study presented by Shamir R. Mehta at TCT 2025, in San Francisco.
Designed by: Elad Asher
Source: PCRonline.com
Why this study – the rationale/objective?
Microvascular obstruction (MVO) after primary PCI occurs in a large minority of STEMI patients, drives larger infarcts and worse outcomes, and has resisted many adjunctive strategies (thrombectomy, intracoronary glycoprotein IIb/IIIa, systemic facilitation) that either failed to help or increased harm.
STRIVE tested whether targeted low-dose intracoronary fibrinolysis (alteplase ≈ 12 % of systemic dose) delivered distal to the culprit lesion could lyse microvascular thrombus, reduce MVO and related events while avoiding systemic bleeding.
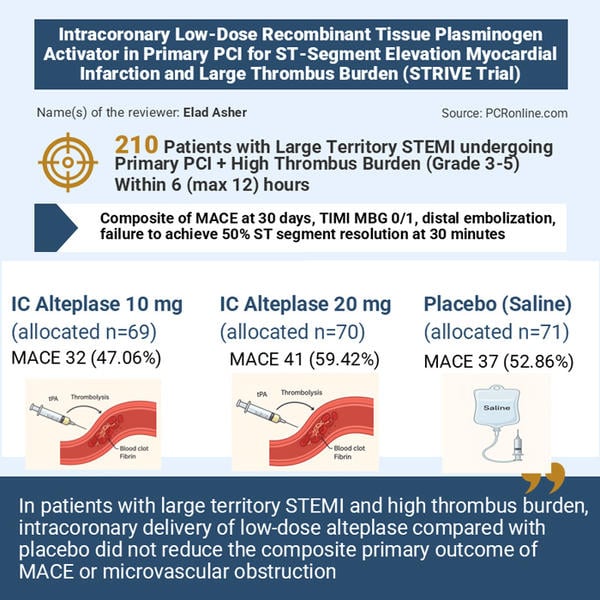
The primary objective was to determine whether adjunctive intracoronary alteplase reduces microvascular obstruction or 30-day major adverse cardiovascular events (MACE) in primary PCI for large-territory STEMI with high thrombus burden.
How was it executed – the methodology?
Design:
Randomised, multicenter, double-blind, placebo-controlled.
Population:
STEMI with high thrombus burden (TIMI thrombus grade 3–5), within 6 (max 12) hours of symptom onset. Median symptom-to-PCI ≈ 3 hours.
Randomisation groups:
Intracoronary alteplase 10 mg (n ≈ 69), 20 mg (n ≈ 70) or placebo (saline, n ≈ 71). Study drug infused over 3 minutes through a catheter positioned distal to culprit lesion after antegrade flow was re-established; PCI then continued per standard practice.
Primary outcome:
Composite of 30-day MACE (CV death, MI, cardiogenic shock, new HF), TIMI myocardial blush grade (MBG) 0/1, distal embolisation, or failure to achieve ≥ 50 % ST-segment resolution at 30 minutes post-PCI.
Power:
Planned n ≈ 201 to detect a 48 % relative risk reduction (assumed event rate 40 %) with 80 % power. Coordinating/core labs blinded; analysis by modified Poisson regression; follow-up vital status 100 % at 30 days.
What is the main result?
No benefit on the primary composite. Combined alteplase groups (n = 137) vs placebo (n = 70): primary outcome occurred in 53.3 % vs 52.9 % (RR 1.00, 95 % CI 0.76–1.31, P = 0.99). Individual dose comparisons likewise showed no significant differences.
No improvement in myocardial blush grade, distal embolisation, or ST-resolution. Secondary and component outcomes were neutral.
Safety:
No increase in major bleeding; clinically relevant non-major bleeding uncommon. However, episodes of ventricular fibrillation (VF) were numerically higher in the alteplase arms (aggregate signal; all VF events were transient and successfully defibrillated).
Reported comparison suggested an elevated RR for VF (≈ 6.9) with wide CI and P ≈ 0.06.
There was no excess mortality.
Critical reading and the relevance for clinical practice
Strengths
- Rigorous randomised, double-blind design with core-lab adjudication and near-complete follow-up.
- Targeted delivery using a distal catheter addresses a mechanistic hypothesis (improve microvascular penetration) that prior trials using guide-catheter delivery (e.g., T-Time) might have missed.
Limitations / potential concerns
- Event assumptions and power: the trial was sized for a very large treatment effect (48 % RRR). If the true effect is modest, STRIVE may have been underpowered to detect it.
- VF signal: increased transient VF during infusion raises safety questions (possible relation to injection speed, alteplase reconstitution, or local myocardial effects); although events were successfully treated and did not translate into higher mortality, the signal is biologically plausible and clinically important.
- Heterogeneity of flow/delivery: suboptimal antegrade flow during infusion might prevent drug reaching microcirculation; though a delivery catheter mitigates this, residual variability remains.
- Neutral consistency with other trials: STRIVE’s negative result aligns with T-Time and meta-analytic trends, reducing the likelihood of a missed benefit.
Should common practice and guidelines be changed?
The negative efficacy result, absence of bleeding penalty, but presence of a safety signal (VF) means there is no net clinical advantage to routine use. Microvascular obstruction remains an unmet need, but this specific intervention does not meaningfully improve patient-level outcomes.
In your view, which aspects of thrombolytic therapy in acute MI should future studies explore further?




1 comment
Nice discussion. My personal experience has been similar and I believe one or two prior studies have shown no benefit as well. Unfortunately I have seen several "desperate" interventionists give IC thrombolytic atop Ticagrelor and IV 2B/3A with resultant intracerebral hemorrhages with disastrous outcomes --definitely a no no !!.