Efficacy and safety of Edoxaban in anticoagulant therapy early after surgical bioprosthetic valve replacement: ENBALV trial
Reported from AHA 2024
Edoardo Zancanaro provides his take on the ENBALV trial presented by Chisato Izumi at AHA 2024 in Chicago.
Why this study – The rationale/objective
The ENBALV (Efficacy and Safety of Edoxaban in Anticoagulant Therapy Early after Surgical Bioprosthetic Valve Replacement) trial was a study aimed at evaluating the safety and efficacy of Edoxaban, a direct oral anticoagulant (DOAC), compared to warfarin, in patients recovering from bioprosthetic valve replacement surgery.
The trial was designed to address the clinical need for safer and more convenient anticoagulation strategies in this patient population, especially during the early post-operative period, when the risk of thromboembolism and bleeding is heightened.
How was it executed – The methodology
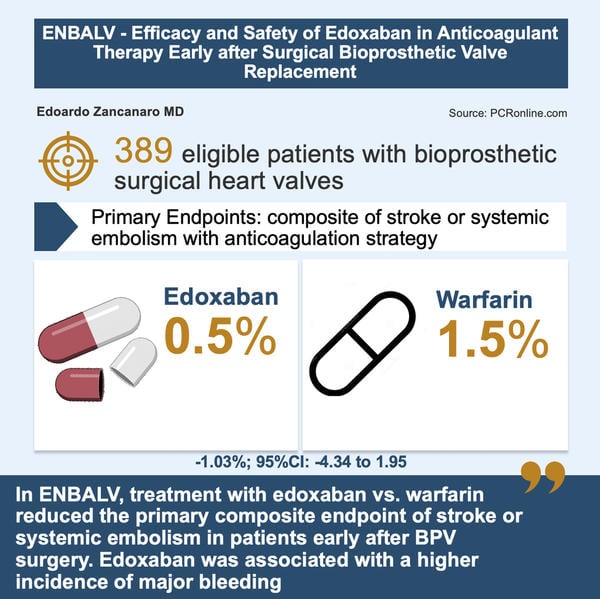
The ENBALV (Japan Registry of Clinical Trials -jRCT- 2051210209. 30 Mar 2022) was an investigator-initiated, multicenter, randomised, open-label trial, in which 389 patients who underwent bioprosthethic valve (BPV) replacement at the aortic and/or mitral position were randomised to Edoxaban (60 mg or 30 mg) once daily or warfarin once-daily for 12 weeks.
Study Endpoints
- Primary endpoint:
- Incidence of stroke or systemic embolism within the 12-week treatment period.
- Secondary endpoints:
- Major bleeding events (according to ISTH criteria).
- Formation of intracardiac thrombus (as assessed by imaging studies).
- Composite outcomes: stroke, systemic embolism, or major bleeding.
- Mortality from any cause.
What are the Main Results?
Efficacy Outcomes
- Primary endpoint (Stroke/Systemic Embolism):
- Edoxaban group: 0.5 % incidence.
- Warfarin group: 1.5 % incidence.
- Intracardiac thrombus formation:
- Edoxaban group: 0 case.
- Warfarin group: 1.0 % incidence.
Safety Outcomes
- Major bleeding:
- Edoxaban group: 4.1 % incidence.
- Warfarin group: 1.0 % incidence.
- Fatal bleeding and intracranial hemorrhage:
- Edoxaban group: 0 case.
- Warfarin group: 1 fatal intracranial hemorrhage.
Composite Outcomes
- Stroke, systemic embolism, or major bleeding:
- Edoxaban group: 4.6 %.
- Warfarin group: 4.5 %.
In ENBALV, treatment with Edoxaban vs. Warfarin reduced the primary composite endpoint of stroke or systemic embolism in patients early after BPV surgery. Edoxaban was associated with a higher incidence of major bleeding.
Critical reading and the relevance for clinical practice
The ENBALV (Efficacy and Safety of Edoxaban in Anticoagulant Therapy Early after Surgical Bioprosthetic Valve Replacement) trial presented very important results whether Edoxaban reduce the endpoints for stroke and systemic embolism.
This results bring two considerations:
The first one is that Edoxaban reduces the need to control INR level, also without the need of blood exams every week. This leads to a better compliance of the therapy and a reduced risk of mis-dosage of the anticoagulation. The second aspect is the reduced need of complication in patients undergoing cardiac surgery operations.
Of interest, Riaz et al showed the real increase in bleeding and/or complications risk with anticoagulation like Warfarin for post-operative management of biological aortic valve replacement. Other studies and meta-analysis went in the same way with similar consideration.
NOAC (New Oral Anticoagulants), like Edoxaban, are known to be well-tolerated in patients with atrial fibrillation or/and in combination with cardiac surgery intervention like mitral valve repair.
Of notice, on the other hand, the present trial showed that despite a favour in results for Edoxaban, Warfarin has less major bleeding, and this may pose the questions on how the major bleeding should be considered on the weigh of patient management in relation to the other endpoints showed.
The clinical impact in the daily practice will be of interest since it is possible that more patients will be more inclined to have a daily therapy without the need of constant blood checking.
In conclusion, ENBALV establishes Edoxaban as an effective and practical anticoagulant that can be used for more variety of patients undergoing cardiac surgery.
The next step would be to address (again) the mechanical valves with NOAC…
Reference:
- Riaz H, Alansari SA, Khan MS, Riaz T, Raza S, Luni FK, Khan AR, Riaz IB, Krasuski RA. Safety and Use of Anticoagulation After Aortic Valve Replacement With Bioprostheses: A Meta-Analysis. Circ Cardiovasc Qual Outcomes. 2016 May;9(3):294-302. doi: 10.1161/CIRCOUTCOMES.115.002696. Epub 2016 May 10. PMID: 27166205; PMCID: PMC5124764.





No comments yet!