The CLEAR SYNERGY (OASIS 9) Trial: A 2x2 factorial randomized controlled trial of colchicine versus placebo and spironolactone versus placebo in patients with myocardial infarction
Reported from AHA 2024
Mirvat Alasnag provides her take on the CLEAR SYNERGY trial presented by Sanjit S Jolly at AHA 2024 in Chicago.
Although secondary preventive measures are often employed following an acute myocardial infarction (MI), residual risk remains a challenge for practitioners. Several trials and registries such as the PLATO-ACS registry noted persistent risk up to 3 years following an event with a strong association with all-cause death and heart failure. (1-2) Inflammatory and metabolic determinants have been postulated.
As such, studies such as CLEAR SYNERGY (OASIS 9) Trial aim to address residual risk. This trial had a unique 2x2 Factorial design. It was a randomized controlled trial exploring the impact of low-dose colchicine (0.5mg daily) versus placebo and spironolactone (25mg daily) versus placebo in patients with myocardial infarction (MI). (3) A total of 7,062 post-MI patients who were within 72 hours of the index percutaneous coronary intervention (PCI) were included. Participants, healthcare providers, research personnel, and outcome adjudicators were blinded to treatment allocation. The primary outcome for colchicine is the first occurrence of the composite of cardiovascular death, recurrent MI, stroke, or unplanned ischemia-driven revascularization. The results of the colchicine intervention were previously presented at TCT 2024. The mean age was 60.5 years 20% of whom were women. Approximately 95% presented with an ST elevation MI (STEMI), and 96% were treated with a drug-eluting stent (DES).
From baseline to 3 months, C-reactive protein (CRP) levels declined in both treatment arms, with significantly larger reductions seen with colchicine. However, the primary endpoint of cardiovascular death, MI, stroke, or ischemia-driven revascularization occurred in 9.1% and 9.3% of colchicine- and placebo-treated patients after a median of 3.5 years, a nonsignificant difference. There was no benefit in any of the individual endpoints although there was a notable reduction in noncardiovascular deaths in the colchicine arm.
These findings are at odds with the previously published COLCOT and LoDoCo2 trials that demonstrated a significant 23% and 31% reduction in cardiovascular events respectively. (4-5)
As a background, spironolactone is associated with a maladaptive response such as vascular inflammation, myocardial cell apoptosis, and fluid retention. Limited data is available on its benefits when used routinely post-MI to prevent heart failure. Trials such as REMINDER and ALBATROSS studies have conflicting results on the benefit of these agents in reducing cardiovascular death and re-hospitalization. (6-7) Therefore, the results of this study weigh into this controversy.
The coprimary outcomes for spironolactone are the composite of the total numbers of cardiovascular deaths or new or worsening heart failure and the first occurrence of the composite of cardiovascular death, new or worsening heart failure, recurrent MI or stroke. For the first primary outcome, there were 183 events in the spironolactone group as compared with 220 events in the placebo group at 3 years (P=0.51).
For the second primary outcome, an event occurred in 280 of 3537 patients (7.9%) in the spironolactone group and 294 of 3525 patients (8.3%) in the placebo group (hazard ratio adjusted for competing risk (P=0.60). Adverse events occurred in 7.2% of the spironolactone group and 6.8% of the placebo group. Therefore, the investigators conclude that following an AMI, spironolactone did not reduce the incidence of death from cardiovascular causes or new or worsening heart failure or the incidence of a composite of death from cardiovascular causes, MI, stroke, or new or worsening heart failure.
It is worth noting that although the majority were STEMIs, 60% were inferior, lateral or posterior where a significant reduction in left ventricular function is not usual. The overall event rate was low introducing a possible Type II error. In addition, the discontinuation rate was significant and it is plausible that the colchicine adverse events in this factorial design contributed to the discontinuation of spironolactone cannot be ascertained.
Finally, whether these results are a class effect cannot be determined particularly with the novel nonsteroidal mineralocorticoid antagonists that appear safer in renal dysfunction with better safety profile.
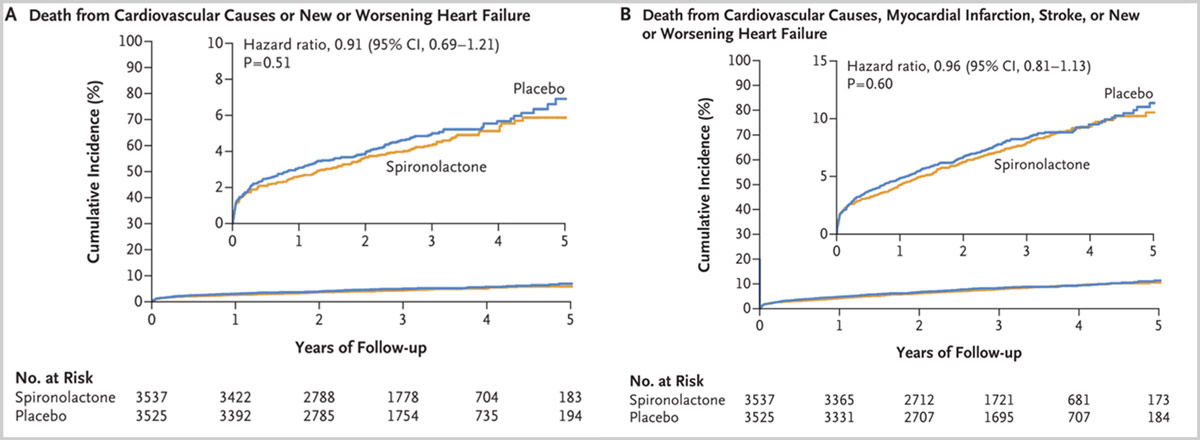
Figures adapted from the NEJM simultaneously published article
DOI: 10.1056/NEJMoa2405923
Primary Outcomes:
(A) Time-to-event curves for death from cardiovascular causes or new or worsening heart failure
(B) Composite of death from cardiovascular causes, myocardial infarction, stroke, or new or worsening heart failure
References:
- A Toso, M Leoncini, M Maioli, S Villani, F Bellandi, PRATO-ACS, Residual risk after acute coronary syndrome: the PRATO-ACS Registry, European Heart Journal, Volume 42, Issue Supplement_1, October 2021, ehab724.2466.
- Jenča D, Melenovský V, Stehlik J, Staněk V, Kettner J, Kautzner J, Adámková V, Wohlfahrt P. Heart failure after myocardial infarction: incidence and predictors. ESC Heart Fail. 2021 Feb;8(1):222-237.
- d'Entremont MA, Lee SF, Mian R, Kedev S, Montalescot G, Cornel JH, Stankovic G, Moreno R, Storey RF, Henry TD, Skuriat E, Tyrwhitt J, Mehta SR, Devereaux PJ, Eikelboom J, Cairns JA, Pitt B, Jolly SS. Design and rationale of the CLEAR SYNERGY (OASIS 9) trial: A 2x2 factorial randomized controlled trial of colchicine versus placebo and spironolactone vs placebo in patients with myocardial infarction. Am Heart J. 2024 Sep;275:173-182.
- Tardif JC, Kouz S, Waters DD, Bertrand OF, Diaz R, Maggioni AP, Pinto FJ, Ibrahim R, Gamra H, Kiwan GS, Berry C, López-Sendón J, Ostadal P, Koenig W, Angoulvant D, Grégoire JC, Lavoie MA, Dubé MP, Rhainds D, Provencher M, Blondeau L, Orfanos A, L'Allier PL, Guertin MC, Roubille F. Efficacy and Safety of Low-Dose Colchicine after Myocardial Infarction. N Engl J Med. 2019 Dec 26;381(26):2497-2505.
- Nidorf SM, Fiolet ATL, Mosterd A, Eikelboom JW, Schut A, Opstal TSJ, The SHK, Xu XF, Ireland MA, Lenderink T, Latchem D, Hoogslag P, Jerzewski A, Nierop P, Whelan A, Hendriks R, Swart H, Schaap J, Kuijper AFM, van Hessen MWJ, Saklani P, Tan I, Thompson AG, Morton A, Judkins C, Bax WA, Dirksen M, Alings M, Hankey GJ, Budgeon CA, Tijssen JGP, Cornel JH, Thompson PL; LoDoCo2 Trial Investigators. Colchicine in Patients with Chronic Coronary Disease. N Engl J Med. 2020 Nov 5;383(19):1838-1847.
- Pitt B, Remme W, Zannad F, Neaton J, Martinez F, Roniker B, Bittman R, Hurley S, Kleiman J, Gatlin M; Eplerenone Post-Acute Myocardial Infarction Heart Failure Efficacy and Survival Study Investigators. Eplerenone, a selective aldosterone blocker, in patients with left ventricular dysfunction after myocardial infarction. N Engl J Med. 2003 Apr 3;348(14):1309-21
- Beygui F, Cayla G, Roule V, Roubille F, Delarche N, Silvain J, Van Belle E, Belle L, Galinier M, Motreff P, Cornillet L, Collet JP, Furber A, Goldstein P, Ecollan P, Legallois D, Lebon A, Rousseau H, Machecourt J, Zannad F, Vicaut E, Montalescot G; ALBATROSS Investigators. Early Aldosterone Blockade in Acute Myocardial Infarction: The ALBATROSS Randomized Clinical Trial. J Am Coll Cardiol. 2016 Apr 26;67(16):1917-27.




No comments yet!