ASSURE DES - Perioperative antiplatelet therapy in patients with coronary stents before non-cardiac surgery
Reported from ESC Congress 2024
Elad Asher provides his take on the ASSURE DES trial presented by Jung-Min Ahn at the ESC Congress 2024 in London.
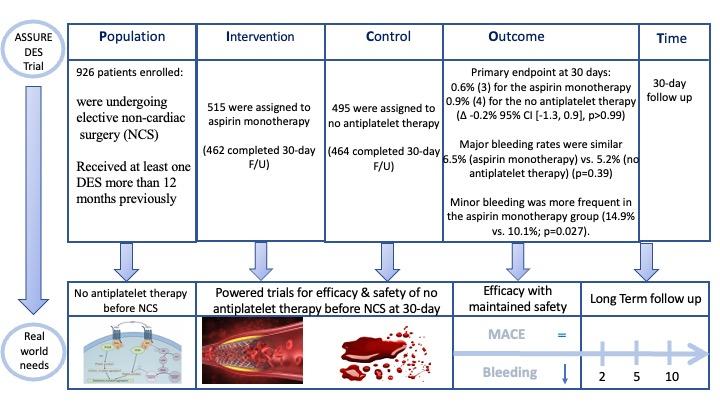
PICOT analysis of ASSURE DES. Courtesy of Elad Asher. Source: PCRonline.com
Why this study? – The rationale/objective
- Up to 20% of patients undergoing PCI with DES need surgical procedures within 2 years of coronary stent implantation.
- Patients undergoing non-cardiac surgery (NCS) are considered at high perioperative cardiovascular risk, facing competing thrombotic and bleeding risks.
- Current guidelines recommend perioperative continuation of aspirin in patients with DES undergoing NCS. However, supporting evidence is limited, especially for contemporary DES.
- The aim of the current trial was to compare a strategy of continuing aspirin monotherapy versus temporarily (5 days) holding all antiplatelet therapy before NCS in patients at least one year after PCI with DES.
How was it executed? – The methodology
- A large-scale, international multicenter, randomized trial
Inclusion Criteria: Patients who had received at least one DES more than 12 months previously and were undergoing elective NCS.
Exclusion criteria: ACS within 1 month; heart failure symptoms (NYHA Fc III-IV); intolerance to aspirin; need for anticoagulation therapy; emergent or cardiac surgery; very high bleeding risk (i.e. intra-cranial surgery, intra-spinal surgery, retinal surgery). - Antiplatelet therapy was discontinued 5 days before surgery and was recommended to be resumed no later than 48 hours after surgery unless contraindicated.
- Patients with aspirin/P2Y12 monotherapy or DAPT were either continue/switch to aspirin alone or stop any antiplatelet agent in a 1:1 randomization fashion.
Primary endpoint:
A composite of death from any cause, MI, stroke or definite stent thrombosis (between 5 days before and 30 days) after NCS
Secondary endpoints:
- Individual component of primary endpoint
- MI
- coronary revascularization
- bleeding (major or minor)
- net adverse clinical events (death; MI; stroke; stent thrombosis; major and minor bleeding)
Statistical considerations: On the basis of POISE-2 trial, the authors assumed that the incidence of the primary composite outcomes at 30 days would be 6% for aspirin monotherapy and 11.5% for the no antiplatelet therapy group. Hence the power calculation was N=900 patients.
Major findings
- In total, 926 patients were analyzed from 30 participating centers in South Korea, India and Turkey. Mean age was 68.5 years and 24% were women.
- On average, DES was implanted 6.3 years before NCS and 84% of subjects had second-generation or newer stents.
- At randomization, 39% of patients were on aspirin monotherapy, 23% on P2Y12 inhibitor Monotherapy and 34% were on DAPT.
- Most surgeries were classified as low-to-intermediate risk for both cardiovascular (89%) and bleeding (88%) events.
- Primary endpoint at 30 days: 0.6% (3) for the aspirin monotherapy group vs. 0.9% (4) for the no antiplatelet therapy group (Δ -0.2% 95% CI [-1.3, 0.9], p>0.99)
- In the aspirin monotherapy group - 2 patients had fatal MI (type 3); 1 had MI (type 2, anemia)
- In the no antiplatelet group – 3 had MI (two had type 1 and required PCI and one type 2, heart failure) and 1 patient had stroke
- Of note, no patient suffered from stent thrombosis
- Major bleeding rates were similar between the groups: 6.5% in the aspirin monotherapy group and 5.2% in the no antiplatelet therapy group (p=0.39). Minor bleeding was more frequent in the aspirin monotherapy group (14.9% vs. 10.1%; p=0.027).
Critical reading and the relevance for clinical practice
The authors of the current trial concluded that continuing aspirin monotherapy did not reduce ischemic events, although it was associated with a modest increase in minor bleeding. Event rates were lower than expected, which may reflect the improved safety profile of contemporary DES.
The low event rate led to the trial being underpowered, hence, the overall findings should be interpreted with caution. Further research in a large-scale, adequately powered study may now be needed, especially in higher-risk patients and where higher-risk surgeries are involved. In the meantime, it seems that a flexible approach to perioperative antiplatelet management may be considered, without compromising patient safety.
Should common practice and guidelines be changed?
The ASSURE DES trial has several limitations: 1) It was underpowered to detect differences between groups; 2) it was an open-label design (reporting bias); 3) there was a limited representation of high-risk procedures (i.e. vascular surgery); 4) patients within the second year after stenting were underrepresented. Nevertheless, 84% of patients had 2nd generation DES and 25% had LM or multivessel PCI before enrolling on the trial.
According to contemporary guidelines it is recommended to continue aspirin peri-operatively (class of recommendation I-B).
What do you think would be the future of aspirin monotherapy in patients undergoing NCS? Do you think we have enough data to support changing the guidelines?
Related publications
- Consider aspirin bridging for non-cardiac surgery in patients with coronary stents treated with oral anticoagulation monotherapy
- Antiplatelet therapy after percutaneous coronary intervention
Latest news from ESC Congress 2024




No comments yet!