07 Nov 2024
Artificial intelligence-based fully automated quantitative coronary angiography versus optical coherence tomography guidance for coronary stent implantation: a multicenter, randomised controlled non-inferiority trial (FLASH)
Reported from TCT 2024
Panos Xaplanteris provides his take on the FLASH trial presented by Jung-Min Ahn at TCT 2024 in Washington.
Why this study – the rationale/objective?
Quantitative analysis of coronary angiograms (QCA) is time-consuming and operator dependent, thus rarely used to guide decisions for percutaneous coronary interventions (PCI) in everyday clinical practice. Artificial intelligence may overcome the limitations of manual QCA.
The FLASH trial was presented at TCT 2024 and reports on the immediate angiographic outcomes of AI-QCA assisted PCI versus optical coherence tomography (OCT) guided PCI.
How was it executed?
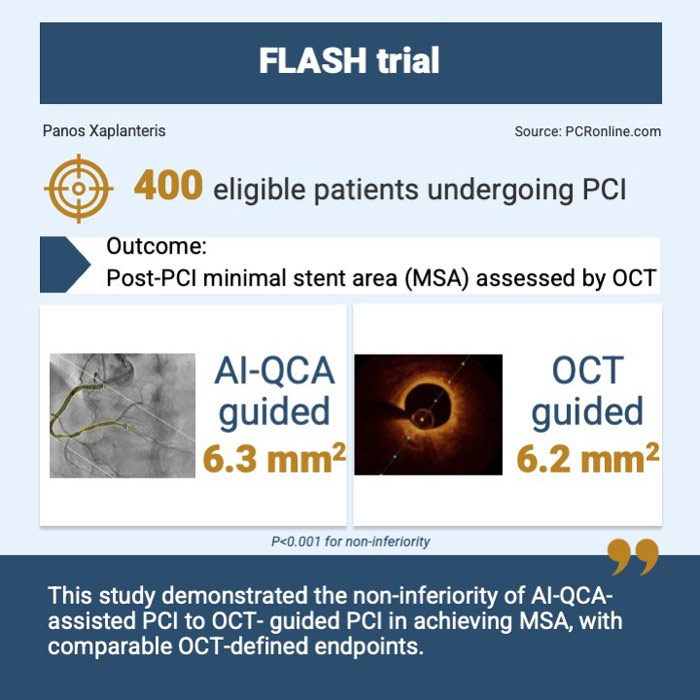
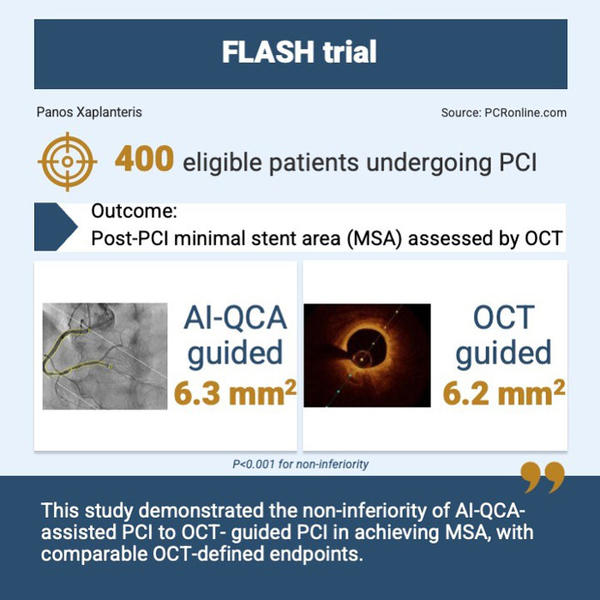
400 patients undergoing PCI from 13 South Korean centers were randomized 1:1 to either AI-QCA assisted or OCT-guided PCI.
Exclusion criteria included left main disease, chronic total occlusion, graft vascular lesion, bifurcation lesions requiring two stent techniques, and any lesion anatomy that would not allow the introduction of an OCT catheter (i.e. severy tortuous or calcified vessels).
The AI-QCA analysis was performed with a proprietary software that automatically analysed the coronary angiography images and calculated minimum/proximal/distal lumen diameters, lesion length and diameter stenosis. According to these parameters, stents oversized by 20 % to the distal reference diameter were chosen. In the OCT group, stent size was selected on the average diameter of external elastic lamina rounded down to the nearest 0.25 mm. All patients in both groups had a final OCT evaluation after PCI.
The primary endpoint was the post-PCI minimal stent area (MSA) as assessed by OCT. A non-inferiority margin of 0.8 mm2 was chosen for AI-QCA assisted PCI versus OCT-guided PCI. Sensitivity analyses for MSA at the proximal and distal stented segment, as well as proximal and distal to a large size branch, were performed. Immediate procedural complications as well as clinical outcomes at 6 months were also recorded and compared between groups.
The mean age of the patients was 65 years, 82 % were men and 40 % presented as an acute coronary syndrome.
What is the main result?
395 patients (199 in the AI-QCA and 196 in the OCT group) were included in the primary endpoint analysis. There were no significant differences between the groups in terms of stent size, balloon size, post-dilation balloon pressure, procedure duration and contrast volume.
The post-PCI MSA for the AI-QCA group was non-inferior (but not superior) to the OCT group (6.3 ± 2.2 mm2 for AI-QCA versus 6.2 ± 2.2 mm2 for OCT: P for non-inferiority < 0.001). Sensitivity analyses showed that MSAs in proximal half segments were comparable between AI-QCA and OCT; this was also the case for distal half segments and if a division by the presence of a large side branch was considered.
Secondary endpoints as stent underexpansion (50.8 % versus 54.6 %, P = 0.48), dissection (15.6 % versus 12.8 %, P = 0.42) and untreated reference segment disease (15.1 % versus 13.3 %, P = 0.61) were comparable between the two groups. However, stent malapposition was more frequent in the AI-QCA group (13.6 % versus 5.6 %, P = 0.007).
Immediate procedural complications as well as clinical events at 6 months were rare and comparable between the two groups.
Critical reading and the relevance for clinical practice
The FLASH trial rekindles the fire of QCA-assisted PCI, a tool that has been underutilised in everyday clinical practice due to its inherent impracticalities, with the twist of a fully automated, online AI algorithm. The immediate vessel-oriented outcomes are non-inferior to those obtained by using the most precise intravascular imaging tool which is OCT.
This has a number of important implications for PCI guidance, the most important being the potential to forego intravascular imaging for non-complex procedures and, thus, decrease costs, use of contrast media and procedural time. Interestingly, however, in the setting of the FLASH trial, such decreases of contrast media and procedural time were not observed.
The AI algorithm provides a rapid, automated and operator-independent vessel analysis in real-time, which is an improvement over manual QCA. However, OCT still remains superior for the detection of stent malapposition owing to its unsurpassed spatial resolution.
Despite the promising results of the AI-QCA assisted PCI, it should be noted that there are still patient subsets in which this approach has not been tested and that are not infrequently met in the cathlab, such as bifurcation lesions, heavily calcified and tortuous vessels, left main and graft lesions.
In conclusion, following the promising results of the FLASH trial and taking into account that in the recent 2024 ESC guidelines for the management of chronic coronary syndromes the use of intravascular imaging (IVUS or OCT) has a I A recommendation, it remains to be seen in the near future which will be the role of AI-QCA for PCI guidance.