Transcatheter aortic valve replacement for asymptomatic severe aortic stenosis: Results of the EARLY TAVR trial
Reported from TCT 2024
Luigi Biasco provides his take on the results of the EARLY TAVR trial, presented by Philippe Genereux at TCT 2024 in Washington.

Current ESC and ACC/AHA guidelines support treatment in symptomatic aortic stenosis (AS) patients with a class I indication. When asymptomatic, adjunctive imaging of laboratory findings such as evidence of impaired left ventricular ejection fraction, very severe aortic stenosis, severe valvular calcifications or markedly elevated BNP levels are required to justify treatment with a class II indication.
While initial data on surgical aortic valve procedures have shown that early surgery is associated with improved outcomes in asymptomatic patients with AS, no data are currently available to support TAVI in asymptomatic AS patients without additional imaging or lab evidence of systolic or diastolic dysfunction.
Ideally, an earlier intervention could prevent progressive left ventricular damage and thus occurrence of heart failure, atrial fibrillation and left ventricular dysfunction.
How was it executed – the methodology?
The EARLY TAVR study is a strategy trial that evaluates an early transcatheter aortic valve replacement with the BEV SAPIEN 3/SAPIEN 3 Ultra THV as compared to an active clinical surveillance in truly asymptomatic patients with severe aortic stenosis with no adjunctive imaging of laboratory findings supporting treatment according to current guidelines indications.
Study population: Patients aged >65 years with severe normal-flow normal-gradient valvular aortic stenosis (bicuspid anatomy allowed) were included in the study. Absence of symptoms related to the severe aortic stenosis was assessed by means of a negative treadmill stress test (positivity defined as presence of syncopal or presyncopal episodes, angina, limiting dyspnea or decreased exercise tolerance, drop in systolic blood pressure or significant ventricular arrhythmias). In patients unable to undergo a treadmill stress test, absence of symptoms was assessed by the treating physician after thorough assessment of patient history.
Methodology
- Treatment arm: transfemoral TAVI with SAPIEN 3/SAPIEN 3 Ultra THV.
- Comparator: Clinical surveillance strategy.
- Patients were randomized 1:1 among treatment and comparator arm.
- Primary endpoint of the study was a composite of death from any cause, stroke, or unplanned hospitalization for cardiovascular causes. Intention-to-treat approach was used for primary end-point analysis.
- In the clinical surveillance group, any aortic-valve intervention within 6 months after randomization was considered for the purposes of the primary end-point.
- According to study design a minimum follow-up of 2 years was planned.
Major findings
Between 2017 and 2021, 901 patients (among 1578 screened among 75 US and Canadian sites) were randomized between early TAVI treatment (n=455) and clinical surveillance arm (n=456). Of the 677 patients excluded the vast majority met a Class I or II indication for TAVI (n=313) while 213 did not fulfill anatomical criteria for a transfemoral TAVI with the investigational device.
Randomized patients had a mean age of 75.8 years, a low STS score (1.8%) and were White in more than 95% of cases.
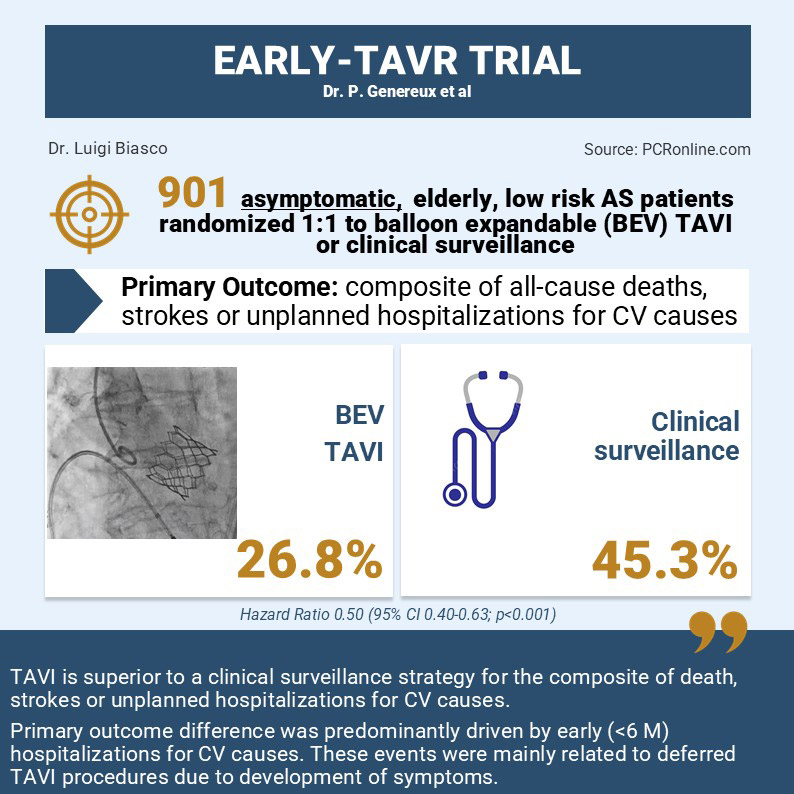
After a mean follow-up of 3.8 years the primary endpoint occurred in 122 (26.8%) in the TAVI group and in 202 (45.3%) in the clinical surveillance group (Hazard Ratio 0.50, 95% CI 0.40-0.63; p<0.001).
When analyzing the individual components of the primary endpoint, no significant differences were evident for the rates of all-cause deaths (HR 0.93; 95% CI: 0.60-1.44). A trend towards a lower incidence of strokes (0.62; 95% CI:0.35-1.10) in the interventional group was emphasized.
The primary endpoint was driven by significant differences in the rate of unplanned hospitalization for cardiovascular causes (TAVI n=95; Clinical surveillance n=186. HR 0.43; 95% CI: 0.33-0.55) with a steep increase of events rate in the clinical surveillance group occurring in the first 6 months after randomization. 105 of the 186 unplanned hospitalizations observed in the clinical surveillance group were related to aortic valve interventions occurring within 6 months after randomization.
One sudden cardiac death, 27 syncopes (17 occurring within 1 year after randomization) and 44 hospitalizations for heart failure or pulmonary edema (24 occurring within 1 year after randomization) were observed in the clinical surveillance group.
Overall, 388 (88%) patients in the clinical surveillance group underwent aortic valve replacement during follow-up. Median time to conversion was 11 months. Conversions were justified by the development of symptoms fulfilling current indications for intervention.
A high safety of transfemoral TAVI in patients enrolled in the treatment arm was mirrored by a low periprocedural event rate (0.9% all-cause mortality, 0.9% stroke, 5.7% new PM implant).
Critical reading and the relevance for clinical practice
The EARLY TAVR trial fills a gap in knowledge as it demonstrates superiority of an early interventional management with TAVI in patients with severe normal flow high gradient aortic stenosis suitable for a transfemoral approach as compared to a watchful waiting strategy with repeated clinical evaluations as recommended by current guidelines.
In order to fully appreciate the true take-home messages of the trial, results and criteria for patient selection have to be interpreted.
First, the clinical surveillance group should not be considered as a medical treatment arm. This group is in fact a delayed intervention group in whom almost 90% of patients effectively received an aortic valve replacement within the study period. 105 out of 388 patients treated by aortic valve replacement were referred for intervention within 6 months from randomization, thus being considered for the purposes of the primary end-point.
Secondly, treadmill exercise tests in patients with severe aortic stenosis might be difficult to adopt in clinical practice, due to the reluctance of physicians regarding safety of testing patients with severe aortic stenosis.
Surely, this trial better clarifies the natural history of severe asymptomatic aortic stenosis showing that, after reaching echocardiographic criteria of severity, symptoms do occur within a relatively short time frame (between 6-12 months).
This finding strengthens the need for adequate surveillance, discussion with patients and family members on the different therapeutic strategies and early initiation of diagnostic work-up in order to get ready for intervention within a reasonable time in case of symptoms occurrence.
Whether the results of this study will change future guideline indications is debated. The lack of impact of an early interventional strategy on all-cause mortality at 4 years reassures about the appropriateness of current practice.






No comments yet!