Cardiac biomarkers in patients with asymptomatic severe aortic stenosis: primary biomarker analysis from the EARLY TAVR Trial
Reported from ACC.25
Elad Asher provides his take on the primary biomarker analysis from the EARLY TAVR Trial presented by Brian R. Lindman at ACC.25 in Chicago.
Designed by Elad Asher. Source: PCRonline.com
Why this study – the rationale/objective?
The EARLY TAVR trial showed that early transcatheter aortic valve replacement (TAVR) was more effective than clinical surveillance (CS) with delayed TAVR in patients with asymptomatic, severe aortic stenosis (AS). Cardiac biomarkers are linked to onset of symptoms, and poorer outcomes after TAVR. However, it is still unclear whether elevated biomarkers can identify asymptomatic patients who are more likely to benefit from early intervention.
The aim of the study was to evaluate whether patients with elevated biomarker levels would have higher event rates and would experience greater benefit from early TAVR compared with those with lower biomarker levels.
How was it executed – the methodology?
It is a prospective, multicenter, open-label, randomised controlled study. It compared upfront TAVR with transfemoral implantation of a SAPIEN 3 or SAPIEN 3 Ultra valve (Edwards Lifesciences) to CS and deferred TAVR in patients with asymptomatic severe AS who met the current guidelines for CS.
Patients were randomised in a 1:1 ratio to early TAVR or CS. Randomised patients were considered part of the intent-to-treat population.
Biomarkers:
Plasma samples were collected at baseline from patients in the EARLY TAVR trial.
- NT-proBNP (proBNP II immunoassay) measurement range of 5-35,000 pg/mL.
- hs-cTnT (Troponin T Gen 5 STAT assay) ranges from 3-10,000 pg/mL.
Both were analysed by the core laboratory and the time from biospecimen collection to TAVR performance in the early TAVR arm was relatively short, with a median of 24 days (IQR 8-43 days).
- Primary endpoint of the EARLY TAVR trial was a non-hierarchical composite of all-cause death, stroke, or unplanned cardiovascular hospitalisation (including aortic valve intervention or reintervention within 6 months of randomisation).
- Secondary composite endpoints included:
- all-cause death, any stroke, or heart failure hospitalization (HFH);
- all-cause death or unplanned cardiovascular hospitalization (including aortic valve intervention or reintervention within 6 months);
- and all-cause death, any stroke, unplanned cardiovascular hospitalization, or intervention/reintervention with advanced signs or symptoms.
Statistical analysis:
For the purpose of analysis, patients were stratified based on their baseline biomarker levels in the following ways:
- the 1st, 2nd, and 3rd tertiles for each absolute biomarker concentration;
- normal vs. elevated biomarker levels;
- and the number of elevated biomarker levels (0, 1, or 2).
Elevated NT-proBNP was defined as ≥ 125 pg/mL for patients under 75 years of age and ≥ 450 pg/mL for those 75 years or older.
Elevated hs-cTnT was defined as ≥ 14 pg/mL for females and ≥ 22 pg/mL for males. The biomarkers were also log-transformed when analysed as continuous variables.
What is the main result?
Major findings
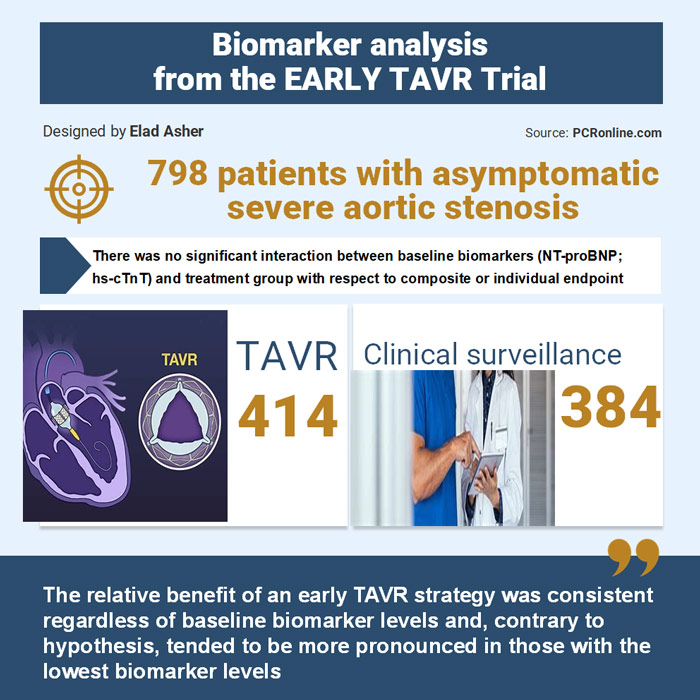
- Biomarker data were available for 798 randomised patients in the intent-to-treat population (414; TAVR arm, 384; CS arm)
- Higher NT-proBNP and hs-cTnT levels were associated with older age, higher STS risk score, inability to perform a treadmill stress test, shorter 6-minute walk distance, a greater burden of atrial fibrillation, and worse renal function.
- Higher NTproBNP levels were associated with lower BMI and less diabetes but more prior stroke.
- Higher hs-cTnT levels were associated with a higher burden of coronary artery disease, including prior MI, and diabetes.
- Higher NT-proBNP and hs-cTnT levels were associated with more severe AS, worse LV systolic and diastolic function, and higher LV mass index.
Biomarkers and event rates:
Higher concentrations of NT-proBNP and hs-cTnT, examined as tertiles, were associated with higher event rates for the trial’s primary and most secondary endpoints among patients in the intention to treat (ITT) population.
In unadjusted and most adjusted analyses, higher NT-proBNP was associated with significantly higher event rates among patients in the ITT population. In contrast, higher hs-cTnT (as a continuous variable) was not associated with higher event rates after adjustment
Two elevated biomarkers, compared to 0, was associated with higher event rates for most endpoints in the ITT population after adjustment; similar associations were seen for several endpoints for those with 1 biomarker elevated
Early TAVR versus clinical surveillance by circulating biomarker concentration:
There were no significant interactions between NT-proBNP nor hs-cTnT biomarker tertiles and treatment group assignment.
The relative benefit of early TAVR, compared with CS, was similar regardless of the biomarker tertile.
While not statistically significant, there was a trend for greater benefit of early TAVR compared with CS for most endpoints examined among those in the lowest biomarker tertile.
Across NT-proBNP and hs-cTnT biomarker tertiles, the difference in absolute event rates between the early TAVR and CS groups and the NNT were fairly similar for most endpoints.
The interaction between hs-cTnT (elevated vs. normal) and treatment group was borderline significant for the composite endpoint of death, stroke, or HFH (interaction p = 0.06), and significant for the composite of death or HFH (interaction p=0.04) and HFH alone (interaction p = 0.03).
Biomarkers and delayed AVR conversion in the clinical surveillance arm:
Increasing NT-proBNP and hs-cTnT tertiles were associated with a higher proportion of an AVR conversion presentation with acute/advanced signs and symptoms, but such a presentation still occurred one-third of the time among those in the lowest biomarker tertiles.
Higher number of counts of elevated biomarkers (0, 1 or 2) was also associated with a somewhat shorter time to AVR conversion.
Critical reading and the relevance for clinical practice
As anticipated, higher levels of NT-proBNP and hs-cTnT were linked to increased event rates for the trial's primary and several secondary endpoints. Notably, the favorable treatment effect of early TAVR, compared to CS, remained consistent across the primary and multiple secondary endpoints, regardless of baseline biomarker levels. This held true whether biomarkers were categorised into tertiles, classified as elevated vs. normal, or based on the number of elevated biomarkers.
Interestingly, there was a numerical trend indicating a lower hazard ratio (suggesting a greater relative benefit of early TAVR) among patients with the lowest, rather than the highest, biomarker levels.
Moreover, NT-proBNP and hs-cTnT did not prove particularly useful in predicting timing of AVR conversion.
The authors also stated that “among those with asymptomatic high gradient severe AS, NT-proBNP and hs-cTnT do not appear to be particularly useful for guiding clinical decision making on the timing of TAVR” and said that there was no difference in the benefit observed from early TAVR across groups based on biomarker concentrations; in fact, the relative benefit of an early TAVR approach was numerically more pronounced among those with the lowest biomarker levels.
Should common practice and guidelines be changed?
The EARLY TAVR trial and other randomised trials testing optimal timing of AVR in patients with asymptomatic severe AS suggest that prompt intervention is superior to clinical surveillance for these patients.
The authors think that the results of this biomarker sub-study reinforce this broad approach and provide evidence that undercuts the guideline recommendation that AVR should be preferentially considered in the subset of asymptomatic patients with severe AS with an elevated BNP.
What do you think would be the future of TAVR in asymptomatic severe AS patients? Do you think we have sufficient data to change the current guidelines?






No comments yet!