29 Mar 2025
Harmonising optimal strategy for treatment of coronary artery diseases – bleeding risk: HOST-BR
Reported from ACC.25
Daniele Giacoppo and Nicola Ammirabile provide their take on the main results of the HOST-BR trial, presented by Hyo-Soo Kim at ACC.25 in Chicago.
Designed by Daniele Giacoppo and Nicola Ammirabile. Source: PCRonline.com
Why this study? – The rationale
Dual antiplatelet therapy (DAPT) with aspirin and a P2Y12 inhibitor is the standard treatment for preventing coronary thrombotic events following percutaneous coronary intervention (PCI) with drug-eluting stent implantation.
However, DAPT unavoidably increases the occurrence of bleeding events, especially in patients with established risk conditions. Current guidelines recommend tailoring the duration of DAPT based on the patient-specific ischaemic and bleeding risk profiles. Since the thrombotic risk is highest in the early post-PCI period, patients at high bleeding risk (HBR) benefit from shorter DAPT durations (1-3 months), achieving improved net composite outcome. In contrast, patients at low bleeding risk have no specific contraindications for more prolonged DAPT regimens (3-12 months). However, there is limited evidence from head-to-head comparisons of DAPT durations guided by the bleeding risk.
The HOST-BR trial sought to evaluate whether shorter DAPT durations could provide noninferior net outcomes and efficacy compared to longer DAPT durations while offering superior safety after stratifying the regimens according to the individual bleeding risk profile.
How was it executed? – The methodology
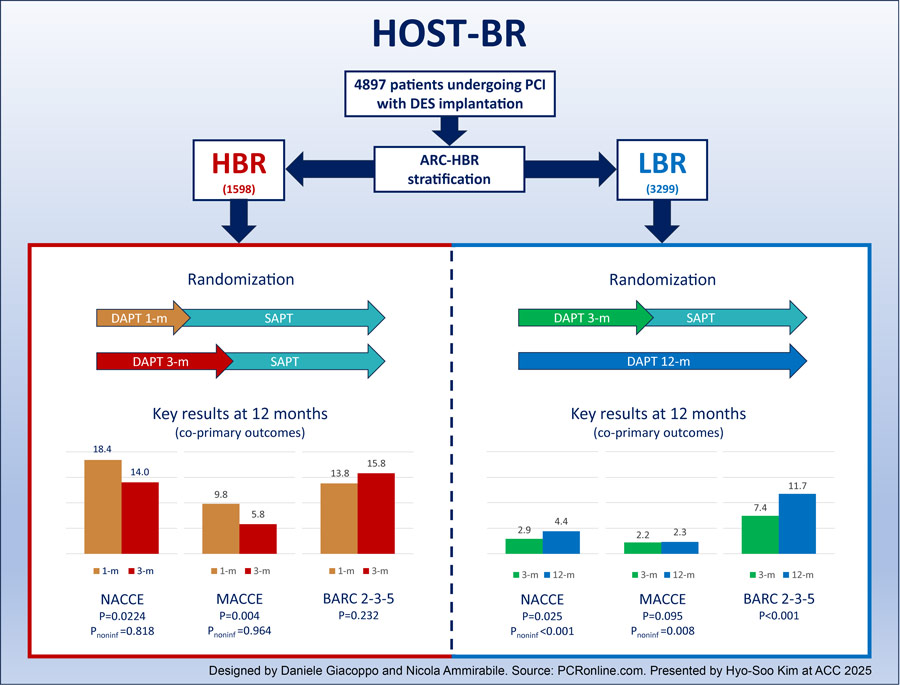
HOST-BR was an investigator-initiated, open-label, multicentre, randomised trial conducted at 53 centres in South Korea, including 4,897 patients undergoing PCI with drug-eluting stents.
Patients were randomised to different DAPT durations after individual stratification based on the Academic Research Consortium HBR criteria (ARC-HBR):
- HBR patients (n = 1,598) were randomised to 1- or 3-month DAPT.
- LBR patients (n = 3,299) were randomised to 3- or 12-month DAPT.
The trial had three co-primary composite endpoints, assessed at 12 months and tested hierarchically:
- Net adverse clinical events (NACE): all-cause death, myocardial infarction, stent thrombosis, stroke, or major bleeding.
- Major adverse cardiac or cerebral events (MACCE): cardiovascular death, myocardial infarction, stent thrombosis, or ischemic stroke.
- Any actionable bleeding: bleeding ARC (BARC) type 2, 3, or 5.
The first two co-primary endpoints – NACE and MACCE – were considered for testing the non-inferiority of shorter DAPT durations (1 month in the HBR cohort and 3 months in the LBR cohort) compared with longer DAPT durations (3 months in the HBR setting). The third co-primary endpoint of BARC 2, 3, or 5 was considered for testing the superiority of shorter DAPT durations compared with longer DAPT durations. Clinical events were adjudicated by an independent committee.
HOST-BR was funded by the manufacturers of the drug-eluting stents used in the trial (Medtronic and Abbott).
What is the main result?
In the HBR cohort, the mean age was 73.8 years, 33.5 % of patients were female, 53.2 % had diabetes, 20.5 % had a history of PCI, and 31.4 % had . The most frequent clinical presentation was stable coronary artery disease (40.6%), while acute myocardial infarction was diagnosed in 30.2 % of patients. At discharge, 97.2 % of patients were prescribed DAPT, with aspirin and clopidogrel used in 92.3 % of regimens.
At 12 months, among HBR patients:
- The incidence of NACE was significantly higher in patients assigned to 1-month DAPT than in those assigned to 3-month DAPT (18.4 % vs. 14.0 %; absolute risk difference [ARD] -4.4 %; hazard ratio [HR] 1.34, 95 % confidence interval [CI] 1.04–1.71, P = 0.022; Pnon-inferiority = 0.818).
- The incidence of MACCE was significantly higher in patients assigned to 1-month DAPT than in those assigned to 3-month DAPT (9.8 % vs. 5.8 %; ARD -4.0 %; HR 1.72, 95 % CI 1.19–2.50, P = 0.004; Pnon-inferiority = 0.964).
- No significant difference in BARC 2, 3, or 5 was observed between 1- and 3-month DAPT (13.8 % vs. 15.8 %, ARD -2.0 %; HR 0.85, 95 % CI 0.66–1.11, P = 0.232).
The analysis of secondary endpoints revealed a higher incidence of cardiovascular death, ischaemic stroke, and target lesion revascularisation in the 1-month DAPT group compared with the 3-month DAPT group. While BARC 2 bleeding was significantly lower in the 1-month DAPT group compared with the 3-month DAPT group, BARC 3 and 5 events did not significantly differ between treatment groups.
In the LBR cohort, the mean age was 63.2 years, 20.9 % of patients were female, 32.3 % had diabetes, 12.9 % had a history of PCI, and 1.8 % had chronic kidney disease. The most frequent clinical presentation was stable coronary artery disease (37.2 %), while acute myocardial infarction was diagnosed in 30.3 %. At discharge, 99.5 % of patients were prescribed DAPT, with aspirin and clopidogrel used in 75.9 % of regimens.
At 12 months, among LBR patients:
- The incidence of NACE was significantly lower in patients assigned to 3-month DAPT than in those assigned to 12-month DAPT. (2.9 % vs 4.4 %; absolute risk difference [ARD] -1.5 %; HR 0.66, 95 % CI 0.46–0.95, P = 0.025; Pnon-inferiority < 0.001).
- The incidence of MACCE in patients assigned to 3-month DAPT was non-inferior to that in patients assigned to 3-month DAPT (2.2 % vs. 2.3 %; ARD -0.1 %; HR 0.98, 95% CI 0.62–1.56, P = 0.95; Pnon-inferiority = 0.008).
- The incidence of BARC 2, 3, or 5 was significantly lower in patients assigned to 3-month DAPT than in those assigned to 12-month DAPT. (7.4 % vs 11.7 %; absolute risk difference [ARD] -4.3 %; HR 0.63, 95 % CI 0.50–0.79, P < 0.001).
The analysis of secondary endpoints revealed that the significant benefit in BARC 2, 3, or 5 associated with 3-month DAPT compared with 12-month DAPT was driven by reductions in both BARC 2 and BARC 3 events. In addition, haemorrhagic stroke was significantly lower in patients assigned to 3-month DAPT than in those assigned to 12-month DAPT.
Critical reading and its relevance for clinical practice
HOST-BR trial is the first randomised trial that integrates the baseline individual bleeding risk differences with varying DAPT durations.
In the HBR cohort, 1-month DAPT was associated with higher incidences of NACE and MACCE compared with 3-month DAPT, while the observed benefit in BARC 2, 3, or 5 bleeding was primarily driven by BARC 2 events.
In the LBR cohort, 3-month DAPT was both non-inferior and superior to 12-month DAPT in terms of NACCE.
MACCE and major individual ischaemic did not significantly differ between treatment groups, and the difference in NACE was essentially driven by a 37 % relative risk reduction in BARC 2, 3, or 5 events. Both BARC 2 and BARC 3 events were significantly reduced in patients assigned to 3-month DAPT compared with those assigned to 12-month DAPT.
While the results of this trial support the use of tailored DAPT regimens according to individual bleeding risk, key factors indicating a different individual ischaemic risk, such as coronary artery disease patterns, PCI complexity, and revascularisation completeness, remain crucial yet missing components in the investigational model. Notably, the HOST-BR trial underscores that HBR is frequently associated with high ischemic risk and high prevalences of major risk factors such as diabetes and chronic kidney disease were observed in the HBR cohort. However, an increasing body of evidence indicates that incorporating angiography and procedural information is essential for optimising personalised DAPT durations.
Although this randomized trial also focuses on patients with LBR, it should be acknowledged that multiple previous randomised clinical trials, predominantly including LBR patients, such as SMART-CHOICE, STOPDAPT-2, GLOBAL-LEADERS / GLASSY, TWILIGHT, TICO, One-Month DAPT, RESET, OPTIMIZE, and REDUCE, have tested DAPT durations of 1 or 3 months with standard 12 month, indicating overall no significant differences in major composite and individual ischaemic endpoints and a benefit of shorter regimes in major bleeding. Similarly, some trials focusing exclusively on the HBR setting, most notably MASTER-DAPT, have evaluated minimalistic regimens, yielding conclusions that differ from those of HOST-BR.
Against this background, considering that almost half of patients in HOST-BR underwent PCI for stable chronic coronary artery disease, employing 12-month DAPT after PCI with drug-eluting stent implantation is often not recommended, except for high ischaemic risk patients. Current European Society of Cardiology and American Heart Association / American College of Cardiology guidelines on chronic coronary syndromes recommend 6 months of DAPT in LBR without high ischaemic risk.
In the HBR cohort of HOST-BR, the reduction in BARC 2, 3, or 5 was essentially driven by the BARC 2 component. The prognostic impact of BARC 2 events is uncertain as some previous studies did not prove an association with survival. However, other studies supported a major prognostic role of BARC 2 events, and the lack of significant differences in BARC 3 and BARC 5 events in the HBR cohort of HOST-BR may be a function of insufficient statistical power to detect significant differences within the narrow timeframe from 1 to 3 months. Conversely, in the LBR cohort of HOST-BR, BARC 3 events and haemorrhagic stroke were significantly reduced in patients assigned to 3-month DAPT compared with those assigned to 12-month DAPT. Considering the established prognostic impact of BARC 3 events and the absence of trade-offs in composite and individual major ischaemic endpoints in the same group of patients, these results are highly relevant.
When interpreting the results of HOST-BR, some considerations are required. In the HBR setting, the expected NACE rates were approximately 50 % lower than those observed, rendering the absolute non-inferiority margin inconsistent with the original assumptions. In addition, while the non-inferiority margin of 2.7 % (30 % of the expected control group rate) may seem reasonable, it is important to note that the incidence of NACE in the experimental group (1-month DAPT) was assumed to be lower than in the control group (3-month DAPT). Although assuming a more favourable expected rate in the experimental group when testing non-inferiority is sometimes employed to reduce conveniently the required sample and facilitate trial success, this approach is conceptually flawed. The non-inferiority hypothesis inherently presupposes no expected difference between groups, while the assumption of a benefit associated with the experimental treatment contradicts this premise. Assessing the other co-primary endpoints, the required sample size computations for MACCE and BARC 2, 3, or 5 were likely constrained by the numerical assumptions used for NACE, leading to some inconsistencies between expected and observed rates. In the LBR setting, the expected rates of NACE, MACCE, and BARC 2, 3, or 5 were slightly higher than those observed, rendering the original hypotheses reasonable. However, as for the HBR setting, the acceptable non-inferiority margin of 1.5 % (30 % of the expected control group rate) should be interpreted against the assumption that the experimental group (3-month DAPT) would have a lower incidence of NACE compared with the control group (12-month DAPT).
In addition, the use of three co-primary endpoints in a fixed-sequence hierarchical order implies that failure to demonstrate non-inferiority for the first composite endpoint of NACE precludes the assessment of the second and third endpoint unless a pre-specified adjustment for multiplicity is applied. Since non-inferiority for NACCE was not demonstrated in the HBR cohort of HOST-BR and no adjustment for multiplicity was planned in the context of fixed-sequence hierarchical testing, the conclusions regarding BARC 2, 3, or 5 in the LBR cohort formally should be considered exploratory.
Finally, in the HBR cohort, a durable-polymer cobalt-chromium everolimus-eluting stent was used, while in the LBR cohort, a durable-polymer cobalt-chromium platinum-iridium inner core zotarolimus-eluting stent was used. Despite these differences, no significant device-related influence on the trial findings is expected. In contrast, the inclusion of only East Asian patients and the predominant use of clopidogrel may limit the generalisability of the results.
Authors





1 comment
Hi, clopidogrel was used in the study. If ticagrelor was P2Y12 inhibitor in 1-3 month arm probably they would get less MACCE.