15-month results of the MASTER DAPT trial
Reported from ESC Congress 2022
Alex Sticchi provides his take on the final results of the MAnagement of high bleeding risk patients post bioresorbable polymer coated STEnt implantion with an abbReviated versus prolonged DAPT regimen

Courtesy of Alex Sticchi @Sticchi_Alex, Source: PCRonline.com
The final results of the MASTER DAPT with the 15 months follow-up have been presented by Prof. Marco Valgimigli at the ESC 2022 in Barcelona.
MASTER DAPT trial proved the non-inferiority for net and major adverse events, and the superiority for bleeding of the abbreviated 1-month dual antiplatelet therapy (AAPT) followed by single antiplatelet therapy (SAPT) until 1 year for patients without a clinical indication for oral anticoagulation therapy (OAC), or 6 months in patients assuming OAC, compared to the standard regimen in high-bleeding risk (HBR) patients undergoing PCI with Ultimaster stent (Terumo, Tokyo, Japan).
This study filled a gap in the current Guidelines where the dual antiplatelet therapy (DAPT) is indicated for 1 to 6 months in patients without OAC, and for 1 week to 1 month with an indication to stop after 6-12 months in patients with OAC.
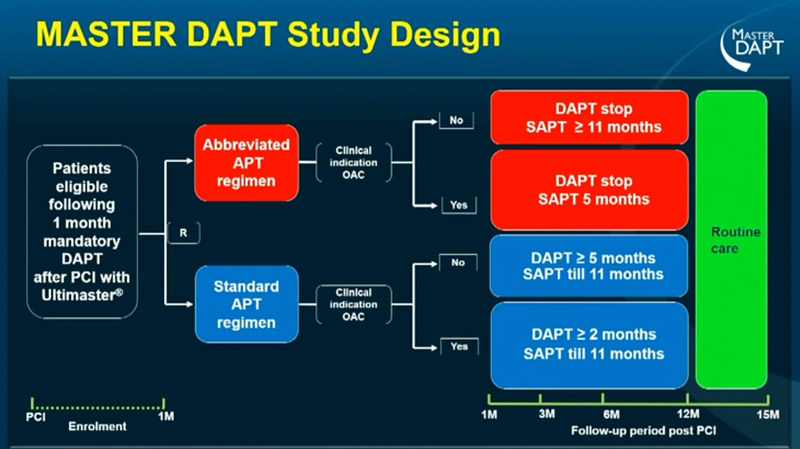
Source: ESC Congress 2022
The randomized allocation lasts from the first month to 1 year and after that time point the patient enters “Routine care”. The physician had the possibility to change the antiplatelet regimen (APT) according to evidence, guidelines and events. At 15 months, a final study visit was performed. This design allowed the MASTER DAPT “to assess the final clinical outcomes at 15 months after PCI, with the rationale of collecting the cumulative treatment effects of an abbreviated versus standard APT when all non-OAC patients were supposed to have discontinued SAPT, irrespective of prior randomization”.
99.9% of participants completed the 15-month Routine Care study visit, and this data is very important for the reliability of the study.
In patients without indication for OAC, the proportion of patients still in DAPT at 15 months was 16.2% compared to the 6.3% of the abbreviated regimen. The same proportion of a threefold was in the group with indication for OAC with 4.2% versus (vs) 1.1% for the standard regimen and the AAPT, respectively. And more than double of patients were in SAPT in the control group.
Furthermore, patients with prior ischemic events were more likely to be in DAPT at 15 months in both groups, irrespectively to the OAC (p<0.001 for the trend in all the groups). On the other side, prior bleeding had a marginal role in the decision to prolong or discontinue the DAPT. Significant predictors of APT use after 15 months at the multivariable analysis were the prior randomization to the abbreviated group in both patients with OAC favouring SAPT vs DAPT and in patients with OAC favouring no-APT vs SAPT. Contemporary, prior myocardial infarction (MI) and revascularization predicted DAPT vs SAPT in patients without OAC, and SAPT vs no-APT in patients with OAC.
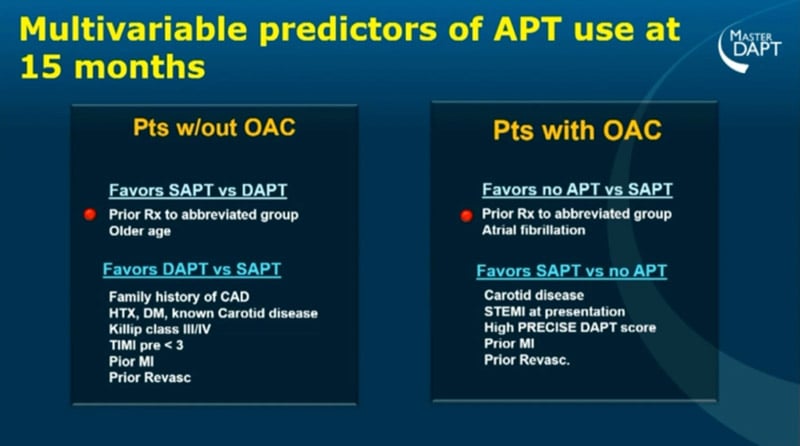
Source: ESC Congress 2022
Net adverse clinical events (NACE: All-cause death, MI, stroke, and major bleeding events defined as BARC 3 or 5) was 9.7% for the standard regimen vs 8.5% for AAPT with a risk difference (RD) of -0.75% and a cumulative Hazard Ratio (HR) of 0.92 (95% CI 0.76-1-12; p=0.40). The NACE rates during the Routine Care period were 1.4% vs 1.8%, numerically favouring the AAPT group.
Major adverse cardiac and cerebral events (MACCE: All-cause death, MI, stroke) were 7.4% for the standard regimen and 6.9% for the AAPT, with a risk difference of -0.51% and an HR of 0.94 (95% CI 0.76-1.17; p=0.58). The MACCE rates showed an incidence of 1.1% and 1.5% for AAPT vs standard regimen, again favouring the experimental strategy.
Finally, major or clinically relevant non-major bleeding (MCB: BARC 2,3 or 5) was 10.7% vs 7.4% in the standard and AAPT groups, respectively. This result leads to a risk difference of -3.25%, even lower than the -2.82% at 12 months, and with a cumulative HR of 0.68 (95% CI 0.56-0.86; p=0.0001).
The secondary endpoints analyses didn’t show significant differences in the several prespecified endpoints except for all-cause death with a similar reduction in both groups.
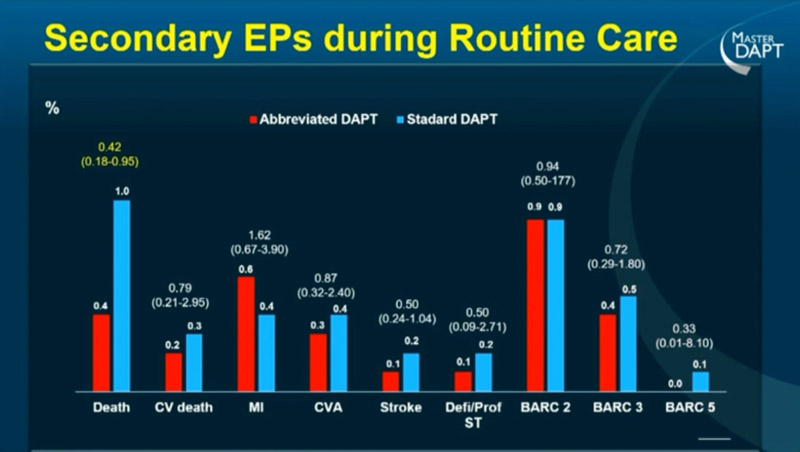
Source: ESC Congress 2022
Despite the several limitations of the study, the authors highlighted as the 15-months results after the routine care phase are only exploratory and hypothesis-generating.
In summary, the MASTER DAPT trial results at 15 months are consistent with the 12-month report and showed no increase in NACE and MACCE outcomes with a contemporary significant reduction in MCB using the AAPT in the HBR population. No rebound phenomenon after discontinuation of APT was recorded in the study.
APT duration has to be defined properly at the PCI discharge using a patient-tailored approach considering the “carry over” effect despite the supposed evidence and guidelines implementation.
Among patients’ physicians, the awareness of prior bleeding is suboptimal compared to the importance attributed to prior ischemic events in the management of APT prolongation or discontinuation.






No comments yet!