TAILORED-CHIP: Tailored antiplatelet therapy for complex high-risk PCI
Reported from ESC Congress 2025
Nicola Ryan provides her take on the results of TAILORED-CHIP presented by Duk-Woo Park at the ESC Congress 2025 in Madrid.
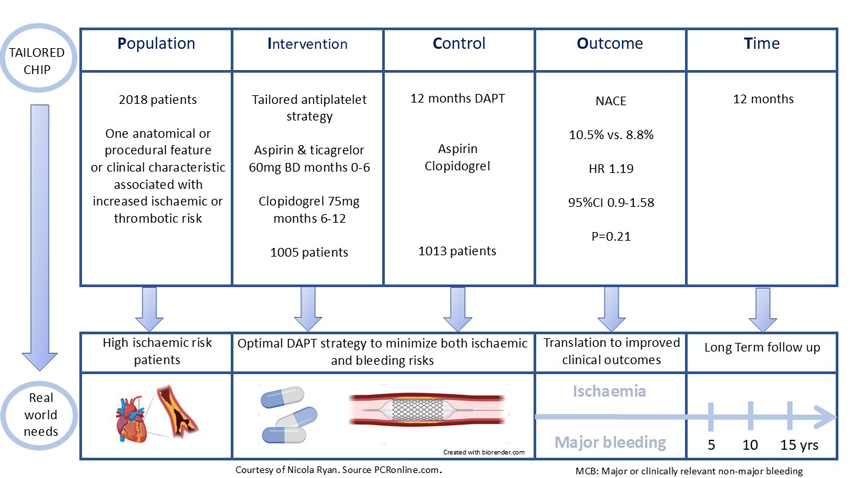
PICOT analysis of TAILORED-CHIP
Courtesy of Nicola Ryan. Source: PCRonline.com
The TAILORED-CHIP trial evaluates the efficacy and safety of temporal modulation of the intensity of platelet inhibition “tailored antiplatelet treatment” versus one year DAPT with aspirin and clopidogrel in patients undergoing complex high-risk PCI.
Why this study – the rationale/objective?
Dual antiplatelet therapy aims to reduce ischaemic risk after PCI but comes with a concomitant increased risk of bleeding. Several trials have focused on bleeding minimisation strategies in patients at high bleeding risk, however, there is limited data with regard to the optimal antiplatelet strategy in patients at high ischaemic risk undergoing complex PCI. Both anatomical and clinical factors place patients at increased ischaemic risk, with the relative risks of ischaemia and bleeding differing over time, i.e. ischaemic risks are typically more common early post-PCI with bleeding risks becoming more prevalent with time. The authors therefore hypothesised that temporal or time dependent modulation of antiplatelet therapy (early escalation and late de-escalation) may be beneficial in high-risk patients undergoing complex PCI.
How was it executed - the methodology?
Patients with at least one high-risk anatomical or procedural factor or clinical characteristic associated with increased risk of ischaemic or thrombotic events undergoing PCI with contemporary drug-eluting stents were eligible for inclusion. Anatomical criteria were left main PCI, complex bifurcation PCI requiring a two-stent technique, chronic total occlusion, severely calcified lesion, diffuse long lesion (≥30mm), multivessel PCI (≥ 2 major epicardial vessels being treated) or complex PCI (≥ 3 planned stents, ≥ 3 lesions treated or expected total stent length > 60mm). Clinical characteristics were medically treated diabetes, CKD (eGFR <60ml/min/1.73m2) or severe LV dysfunction (EF <40%). Key exclusion criteria included ACS, long-term use of anticoagulants and high bleeding risk conditions.
Patients were randomised 1:1 to tailored antiplatelet strategy (early escalation <6 months: ticagrelor 60mg BD and aspirin, late de-escalation 6-12 months clopidogrel monotherapy) or DAPT with aspirin and clopidogrel for twelve months. Patients were followed up clinically at 1, 3, 6 and 12 months.
- The primary outcome was net adverse clinical events: a composite of all-cause death, MI, stroke, stent thrombosis, unplanned urgent revascularisation or clinically relevant bleeding (BARC Type 2,3 or 5) at 12 months.
- Secondary outcomes included
- The components of the primary outcome
- A composite of major ischaemic events (death from any cause, MI, stroke, stent thrombosis, or unplanned urgent revascularisation)
- A composite of hard clinical endpoints (death from any cause, MI or stroke)
- Safety outcomes (clinically relevant bleeding, major bleeding, fatal bleeding or any major or minor bleeding)
- Bleeding events were additionally classified according to BARC, GUSTO, TIMI and ISTH criteria
What is the main result?
From February 2029 to January 2024, 2,710 patients in 24 Korean centres were screened for inclusion in the trial. Of the 2,018 patients included 1,005 were randomised to tailored antiplatelet therapy and 1,013 to DAPT. The majority of patients, 83% were male with a mean age of 64 years. Traditional CVRFs were common with a fifth of the population having undergone prior PCI. Almost all patients (99.6%) had at least one high-risk anatomical or procedural criteria, with at least one high-risk clinical condition present in almost half the population. In keeping with the South Korean population studied, there was high use of intravascular guided imaging PCI (71%).
- Total NACE was similar between groups, tailored antiplatelet strategy 10.5% vs. DAPT 8.8% (HR 1.19, 95%CI 0.90-1.58, p=0.21) at 12 months
- The incidence of major ischaemic events did not differ between groups 3.9% tailored antiplatelet strategy vs. 5.0% DAPT (HR 0.78 95%CI 0.52-1.19, p=0.25
- Hard clinical endpoints were numerically but not statistically lower with a tailored antiplatelet strategy 3.3% vs 4.9% (HR 0.68, 95%CI 0.43-1.05, p=0.08)
- The tailored antiplatelet strategy had a higher incidence of clinically relevant bleeding 7.2% vs. 4.8% (absolute difference 2.45%, 95%CI 0.37%-4.53%, p=0.002)
- Major bleeding was similar between groups, irrespective of the criteria used.
Critical reading and the relevance for clinical practice
The results of this multicentre randomised controlled trial comparing a tailored antiplatelet strategy with temporal modulation of antiplatelet intensity to 12 months DAPT in high ischaemic risk patients did not reduce the primary outcome of net adverse events at 12 months. In terms of both major ischaemic events and hard clinical endpoints (a composite of death, MI & stroke), there were numerically lower event rates in the tailored antiplatelet strategy group, which did not meet statistical significance. However, the tailored antiplatelet strategy group had significantly higher clinically relevant bleeding events (BARC Type 2, 3 or 5) with no differences in major bleeding (BARC Type 3 or 5).
A landmark analysis at 6 months demonstrated that majority of the clinically relevant bleeding events in the tailored antiplatelet strategy group occurred within the 6-month escalation strategy period. These bleeding events were predominantly BARC Type 2 events or “minor bleeding” however carry implications for patient’s quality of life. During the six-month de-escalation period, there was a borderline reduction in bleeding with clopidogrel monotherapy (0.7% vs 1.7%, HR 0.42, 95%CI 0.17-0.99, p=0.05). This landmark analysis supports the safety of P2Y12 monotherapy at 6 months post-PCI, even in high ischaemic risk patients undergoing complex PCI.
The majority of ischaemic events appeared to occur within the first month, this, along with the lower rates of the “hard endpoint” of the composite of death, MI & stroke in the tailored antiplatelet strategy group, suggests that a shorter period of escalation may be worthy of investigation in future randomised control trials. The Twilight trial, which included patients with either ischaemic or bleeding risk factors, demonstrated a reduction in bleeding without an ischaemic penalty with three months of DAPT with aspirin and ticagrelor followed by ticagrelor monotherapy (1).
As with all trials, there were a number of limitations, importantly the choice of a combined bleeding and ischaemic endpoint along with a lower than expected event rate potentially led to bias towards the less potent P2Y12 strategy. The study was carried out in an East Asian population using the lower dose ticagrelor 60mg BD, limiting its generalisability to a broader population. However, the ALPHEUS trial using ticagrelor 90mg BD compared to clopidogrel post-elective PCI in a European population demonstrated increased minor but no increased major bleeding with ticagrelor in this group (2). Finally, there were very few women (17%) enrolled in the trial and patients at high bleeding risk were excluded.
In light of the TAILORED-CHIP trial results, it would seem that the current ESC guideline recommendation of six months DAPT with aspirin and clopidogrel post PCI in patients with chronic coronary syndrome should remain the default (3).
References
- Mehran R, Baber U, Sharma SK, Cohen DJ, Angiolillo DJ, Briguori C, et al. Ticagrelor with or without Aspirin in High-Risk Patients after PCI. New England Journal of Medicine. 2019 Nov 21;381(21):2032–42.
- Silvain J, Lattuca B, Beygui F, Rangé G, Motovska Z, Dillinger JG, et al. Ticagrelor versus clopidogrel in elective percutaneous coronary intervention (ALPHEUS): a randomised, open-label, phase 3b trial. The Lancet. 2020 Nov 28;396(10264):1737–44.
- 2024 ESC Guidelines for the management of chronic coronary syndromes | European Heart Journal | Oxford Academic [Internet]. [cited 2025 Sep 1]. Available from: https://academic.oup.com/eurheartj/article/45/36/3415/7743115
Latest news from ESC Congress 2025




No comments yet!