External applicability of the ISCHEMIA trial: an analysis of a prospective nationwide registry of patients with stable coronary artery disease
Selected in EuroIntervention Journal by R. Simpson , K. De Silva
This study aimed to delineate the external validity of the ISCHEMIA trial by applying the ISCHEMIA inclusion and exclusion criteria to an established cohort of patients with stable coronary disease in an observational setting.
References
Authors
Leonardo De Luca, Massimo Uguccioni, Jennifer Meessen, Pier Luigi Temporelli, Fabrizio Tomai, Francesco Mario De Rosa, Enrico Passamonti, Dario Formigli, Carmine Riccio, Domenico Gabrielli, Furio Colivicchi, Michele Massimo Gulizia, Gian Piero Perna
Reference
10.4244/EIJ-D-20-00610
Published
December 2020
Link
Read the abstractReviewers
Our Comment
Why this study? – the rationale/objective
The ISCHEMIA trial1 showed that an initial invasive strategy did not decrease the incidence of CV events or death compared to optimal medical therapy alone in patients with stable coronary disease and demonstrable moderate to severe ischemia.
However, randomized clinical trials frequently produce skewed patient populations which may not be representative of ‘real-world’ unselected patients.
Understanding the external applicability of RCT data is important to allow trial evidence, to appropriately interpret, and to ensure optimal, patient-centered, management decisions.
In this regard, De Luca at al2 aimed to delineate the external validity of the ISCHEMIA trial by applying the ISCHEMIA inclusion and exclusion criteria to an established cohort of patients with stable coronary disease in an observational setting.
How was it executed? – the methodology
The authors applied the original inclusion and exclusion criteria of the ISCHEMIA trial to the Stable Coronary Artery Disease RegisTry (START), a national, Italian database of patients with stable coronary disease. This created three groups: ‘ISCHEMIA-Like’, ‘ISCHEMIA Not-Included/Unclassifiable’ and ‘ISCHEMIA Excluded’.
A statistical comparison was then undertaken between the ISCHEMIA-like and ISCHEMIA not-included/unclassifiable groups. Included in the START registry were patients with typical or atypical chest pain, documented ischaemia on a stress test, previous revascularisation (PCI and CABG), prior acute coronary syndrome and elective admissions for revascularisation including staged procedures. Patients were defined as being on OMT (as similarly defined in the ISCHEMIA trial) if they were taking an anti-platelet, beta-blocker, and statin at the maximum tolerated dose.
The primary outcome was MACE as a composite if death from CV causes, MI, hospitalization for unstable angina and heart failure at one-year follow up, with a composite secondary outcome of CV mortality and MI at one-year. All patients included were followed-up by clinic visits or telephone calls. During this interview, patients also underwent an EQ-5D-5L quality of life health questionnaire (a general health care and lifestyle questionnaire comprising of five domains). Scores in each domain are ranked as a visual analogue, from 0 (worst score) to 100 (best score).
What is the main result?
Of the registry of 5,070 patients, 84.7 % did not fulfill ISCHEMIA inclusion criteria (ISCHEMIA-Not included/Unclassifiable), 11.5 % met exclusion criteria (ISCHEMIA-Excluded), leaving 3.8 % patients that met inclusion criteria (ISCHEMIA-Like).
At one-year primary outcome was just 0.5 % in the ISCHEMIA-Like as compared to 3.3 % other patients (p = 0.03). Secondary outcome was 0.5 % in ISCHEMIA-Like when compared to 1.4 % in remaining patients (p = 0.1). The ISCHEMIA-Like group had lower rates of malignancy, atrial fibrillation, revascularisation and heart failure and a higher mean left ventricular ejection fraction (57.9 % vs 53.8 %, p = < 0.0001) than those in the ISCHEMIA-Not included/Unclassifiable. The ISCHEMIA-like group also had a lower incidence of multi-vessel coronary disease compared with patients in the ISCHEMIA-excluded group. The uptake of OMT were similar in both groups (over 65 %, p = 0.22). The quality-of-life questionnaire was completed in 96 % of patients, with a median general health status score of 75 out of 100.
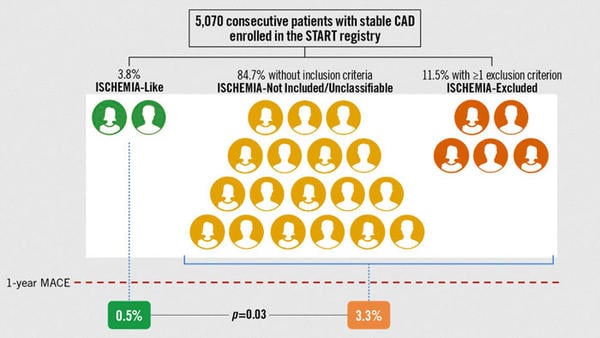
Proportion of patients with stable CAD enrolled in the START registry with or without ISCHEMIA criteria and their rate of MACE at 1 year.
Source: EuroIntervention Journal
Critical reading and the relevance for clinical practice:
The analysis of the START registry seems to suggest that, in a real-world national repository of patients, there is limited external generalizability of the ISCHEMIA trial. Within the small group identified as an ISCHEMIA-like cohort in this analysis, there was a very low risk of MACE (0.5 % at one-year) and a good quality of life when compared to those patients that were not categorized as eligible, whom had a greater risk of MACE (3.3 % at one-year) and poorer QOL outcomes. The MACE rate observed in the ISCHEMIA-like arm was significantly lower than those observed in the ISCHEMIA trial (5.3 % and 3.4 % in the revascularization and OMT arms respectively).
However, the analysis and subsequent inferences suggesting limited external validity of the ISCHEMIA trial should be interpretated with a degree a caution and noting a number of caveats.
- Firstly, the ISCHEMIA trial mandated that all patients underwent non-invasive stress testing; this is not the case for enrollment in the START database, which rendered many patients ineligible, accounting for the high number of ISCHEMIA-Excluded/Unclassifiable patients.
- Secondly, the severity of coronary disease observed in the ISCHEMIA-like cohort was significantly reduced, with only 12 % having three-vessel CAD compared to 42 % in ISCHEMIA. Consequently, this could have led to a greater overall ischaemic burden in the ISCHEMIA trial patients which may have led to the higher observed event rates.
- Thirdly, the current analysis used a universal definition of myocardial infarction for endpoint analysis, which differed to the strict periprocedural myocardial infarction definition used in the ISCHEMIA trial; and may have been one of the contributing factors in the differences observed in MACE rates between the ISCHEMIA trial and the START ISCHEMIA-like sub-group.
- Finally, the current analysis was undertaken following a much shorter follow-up time point of 1-year compared to a median of 3.2 years in ISCHEMIA, meaning the event rates were not directly comparable as there was less time for accrual of events in the current analysis.
In summary, the analysis of the START registry has provided an interesting context of how the ISCHEMIA trial may be externally applied in a real-world observational cohort. However, definitive conclusions about ISCHEMIA’s applicability to contemporary clinical practice cannot be affirmed from this current study. Further insights with a more matched population would be additive to the literature and provide further guidance in this space.
References
- Maron DJ et al; ISCHEMIA Research Group. Initial Invasive or Conservative Strategy for Stable Coronary Disease. N Engl J Med. 2020;382:1395-407.
- De Luca L et al; External applicability of the ISCHEMIA trial: an analysis of a prospective, nationwide registry of patients with stable coronary artery disease, Eurointervention, Volume 16, No 12, 16:e966-e973. DOI: 10.4244/EIJ-D-20-00610




No comments yet!