Ticagrelor monotherapy in patients at high bleeding risk undergoing percutaneous coronary intervention: TWILIGHT-HBR
Selected in European Heart Journal by A. N. Calik , E. Ilkay Yuce
To further investigate the treatment effects of ticagrelor monotherapy compared with ticagrelor plus aspirin in a contemporary HBR population, a pre-specified analysis of the TWILIGHT trial, TWILIGHT-HBR, was conducted by using the Academic Research Consortium (ARC) criteria for HBR.
References
Authors
Javier Escaned, Davide Cao, Usman Baber, Johny Nicolas, Samantha Sartori, Zhongjie Zhang, George Dangas, Dominick J Angiolillo, Carlo Briguori, David J Cohen, Timothy Collier, Dariusz Dudek, Michael Gibson, Robert Gil, Kurt Huber, Upendra Kaul, Ran Kornowski, Mitchell W Krucoff, Vijay Kunadian, Shamir Mehta, David J Moliterno, E Magnus Ohman, Keith G Oldroyd, Gennaro Sardella, Samin K Sharma, Richard Shlofmitz, Giora Weisz, Bernhard Witzenbichler, Stuart Pocock, Roxana Mehran
Reference
10.1093/eurheartj/ehab702
Published
October, 18 2021
Link
Read the abstractReviewers
Our Comment
Why this study? – the rationale/objective
The potent P2Y12 inhibitors, prasugrel and ticagrelor, demonstrated superior ischaemic protection than clopidogrel among acute coronary syndromes (ACS) patients on background aspirin therapy. Nonetheless, this beneficial effect is mitigated because of the increased bleeding risk with their use, which is of utmost importance as thrombotic complications after PCI.
More patients with high bleeding risk (HBR) are now undergoing PCI with more frequently used advanced technological devices, such as ultra-thin strut stents and intravascular imaging tools. These conditions made standard medication with dual antiplatelet therapy (DAPT) clinically less desirable and led the physicians to find novel antiplatelet treatment strategies after PCI, including shortening the DAPT duration by dropping aspirin whenever possible. However, the use of these strategies is not straightforward because HBR patients have generally been excluded or underrepresented in clinical research on DAPT, and they often are at increased risk of ischaemic events as well.
The TWILIGHT trial tested the safety of DAPT de-escalation to ticagrelor monotherapy starting after an adverse event-free 3 month period following PCI in patients at high thrombotic and/or bleeding risk according to a list of clinical and angiographic criteria. The results showed that ticagrelor monotherapy after a short course of DAPT is an effective and safe bleeding avoidance strategy among high-risk patients undergoing PCI.
To further investigate the treatment effects of ticagrelor monotherapy compared with ticagrelor plus aspirin in a contemporary HBR population, a pre-specified analysis of the TWILIGHT trial, TWILIGHT-HBR, was conducted by using the Academic Research Consortium (ARC) criteria for HBR.
How was it executed? - the methodology
TWILIGHT was a randomized, placebo-controlled trial conducted at 187 sites in 11 countries. Patients undergoing successful PCI with a drug-eluting stent were eligible for study enrolment if they satisfied at least one clinical and one angiographic criterion associated with a high risk of ischaemic or bleeding events.
After the index PCI, all enrolled patients received ticagrelor (90mg twice daily) and aspirin (81–100mg daily). At 3 months, patients without major bleeding or ischaemic events were randomized 1:1 in a double-blind fashion to aspirin or placebo for an additional 12 months in addition to ticagrelor.
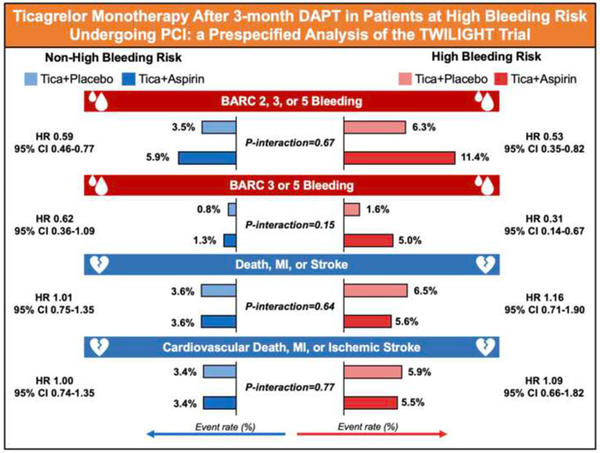
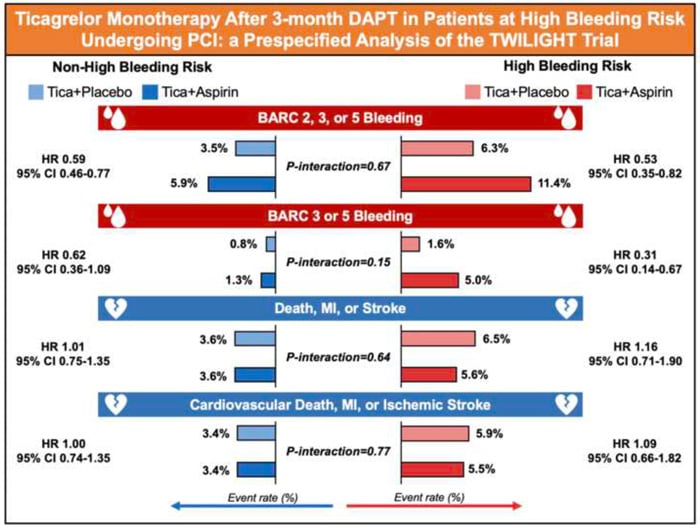
The primary endpoint was Bleeding Academic Research Consortium (BARC) type 2, 3, or 5 bleeding up to 1 year after randomization. The key secondary endpoint was the composite of all-cause death, MI, or stroke.
Moreover, BARC 3 or 5 bleeding and the composite of cardiovascular death, MI, or ischaemic stroke were considered as outcomes of interest for this analysis in line with the recommendations of the ARC on HBR trial design principles.
Patients were considered HBR if they fulfilled at least one major or two minor criteria defined by the ARC-HBR consensus statement.
Major criteria available for analysis were severe or end-stage CKD, haemoglobin < 11 g/dL, moderate or severe thrombocytopenia, previous major bleeding, and liver disease.
Minor criteria included age > 75 years, haemoglobin 11-13 g/dL for men, 11-12 g/dL for women, and non-steroidal anti-inflammatory drug use.
In the primary pre-specified analysis, the treatment effects of ticagrelor monotherapy vs. ticagrelor plus aspirin were evaluated according to HBR status.
What is the main result?
After applying exclusion criteria, the final study cohort included 6,178 patients, of whom 1,064 (17.2 %) were HBR.
Among HBR patients, moderate CKD and age > 75 years were the two most common minor criteria (55.4 % and 49.4 %, respectively), while haemoglobin < 11 g/dL was the most common major criterion (24.2 %).
The primary endpoint of BARC 2, 3, or 5 bleeding occurred in 93 patients (8.9 %) in the HBR group and in 237 patients (4.7 %) in the non-HBR group (HR 1.95, 95 % CI 1.54–2.48; P < 0.001).
In HBR patients, there was a significant reduction in the incidence of BARC 2, 3, or 5 bleeding randomized to ticagrelor plus placebo compared with those randomized to ticagrelor plus aspirin (6.3 % vs. 11.4 %; HR 0.53, 95 % CI 0.35–0.82; P = 0.004). Treatment effects on BARC 2, 3, or 5 bleeding were also consistent among non-HBR patients (3.5 % vs. 5.9 %; ARD -2.3 %, 95 % CI -3.5 % to -1.2 %; HR 0.59, 95 % CI 0.46–0.77; P < 0.001).
The incidence of BARC 3 or 5 bleeding was significantly higher in HBR than non-HBR patients (3.4 % vs. 1.0 %; HR 3.30, 95 % CI 2.15– 5.07; P < 0.001). Ticagrelor plus placebo resulted in lower rates of BARC 3 or 5 bleeding in both HBR (1.6 % vs. 5.0 %; HR 0.31, 95 % CI 0.14–0.67, P = 0.003) and non-HBR patients (0.8 % vs. 1.3 %; HR 0.62, 95 % CI 0.36–1.09, P = 0.098; P interaction = 0.148).
A total of 66 (6.1 %) key secondary endpoint events (all-cause death, MI, or stroke) occurred in HBR patients as compared with 181 (3.6 %) in those without HBR (HR 1.70, 95 % CI 1.27–2.26; P < 0.001). However, there were no significant differences between treatment arms among HBR and non-HBR patients with respect to the thrombotic events (all-cause death, MI, or stroke were).
It is important to note that The TWILIGHT-HBR analysis was underpowered to detect clinically relevant differences in ischaemic events, and findings must be seen as hypothesis-generating.
Source: European Heart Journal
Critical reading and the relevance for clinical practice
TWILIGHT-HBR pre-specified subgroup analysis of patients with at least a 4 % risk of BARC bleeding 3 (= overt bleeding plus haemoglobin drop at least 3 g per dL, or cardiac tamponade, or intraocular bleed compromising vision, or need of any transfusion, intravenous vasoactive agents, surgical intervention to stop bleeding) or 5 (fatal bleeding) or at least a 1 % risk of intracranial bleeding within 12 months after the index PCI has provided essential insights for selecting antiplatelet therapy strategy in HBR patients.
This pre-specified analysis of the TWILIGHT study demonstrated:
- HBR patients experienced higher rates of bleeding and ischaemic events than non-HBR patients, with a risk proportional to the number of fulfilled ARC-HBR criteria.
- Ticagrelor monotherapy lowered the risk of clinically relevant BARC 2, 3, or 5 bleeding without increasing ischaemic events, including death, MI or stroke, irrespective of HBR status.
- The absolute reduction in major bleeding complications associated with ticagrelor monotherapy was more pronounced in HBR than non-HBR patients.
The results revealed that the incidence of the primary BARC 2, 3, or 5 bleeding endpoint was nearly doubled in HBR vs non-HBR patients, while BARC 3 or 5 bleeding was increased by more than three times. Even in these higher-risk cohorts who meet the ARC-HBR definition, the treatment effects of ticagrelor monotherapy reported in the main trial are preserved.
In short, these findings highlight the role of 3-month DAPT followed by ticagrelor monotherapy as a safe and effective bleeding avoidance strategy among HBR patients enriched with high ischaemic risk features who undergo PCI with a drug-eluting stent.







No comments yet!