21 Jan 2022
30-day outcomes following transfemoral transseptal transcatheter mitral valve replacement: Intrepid TMVR early feasibility study results
Selected in JACC: Cardiovascular Interventions by L. Biasco
The study by Zahr et al. reports the results of an interim analysis assessing procedural and clinical outcomes at 30-day in 15 patients treated with the newly developed Medtronic Intrepid 35-F trans-femoral transcatheter mitral valve replacement (TMVR) system.
References
Authors
Firas Zahr, Howard K. Song, Scott M. Chadderdon, Hemal Gada, Mubashir Mumtaz, Timothy Byrne, Merick Kirshner, Tanvir Bajwa, Eric Weiss, Susheel Kodali, Isaac George, John Heiser, William M. Merhi, Jeremy J. Thaden, Angie Zhang, D. Scott Lim, Michael J. Reardon, David H. Adams, Michael J. Mack, and Martin B. Leon
Reference
J Am Coll Cardiol Intv. 2022 Jan, 15 (1) 80–89
Published
January 2022
Link
Read the abstract
Reviewer
Latest contributions
TAVI complications - Part 5 Unusual structural interventions: infective endocarditis, ventricular septal rupture & hypertrophic obstructive cardiomyopathy Tendyne transcatheter mitral valve system in patients with severe mitral annular calcification: one-year outcomes from the SUMMIT Severe MAC cohortMy Comment
Why this study – the rationale/objective?
The study by Zahr et al. reports the results of an interim analysis assessing procedural and clinical outcomes at 30-day in 15 patients treated with the newly developed Medtronic Intrepid 35-F trans-femoral transcatheter mitral valve replacement (TMVR) system.
How was it executed? - the methodology
Patients were enrolled from February 2020 to May 2021 among 9 high volume, academic, US centers.
Main inclusion criteria were:
- presence of a moderate or severe symptomatic mitral regurgitation (MR) either of primary or secondary etiology,
- inappropriate or high risk for conventional surgery and with anatomy better treated by TMVR than by transcatheter edge-to-edge mitral valve repair (TEER).
Key exclusion criteria were:
- high predicted risk of operative mortality or irreversible major morbidity (heart team assessed),
- presence of anatomical contra-indication to Intrepid device (mainly related to the annular size),
- high risk for left ventricular outflow tract obstruction,
- severe tricuspid regurgitation, severe right or left ventricular dysfunction (LVEF < 25 %, or LVEF 25-30 % when associated with left ventricular end diastolic volume index (LVEDVi) > 120 mL/m2).
Procedural, clinical, and echocardiographic endpoints were defined according to MVARC criteria and adjudicated by an external clinical events committee.
What is the main result?
Median age of study patients was 80 years.
Patients were at intermediate-to-high surgical risk as assessed by the STS score (median 4.7 %).
Primary MR was present in the majority of patients (10 out of 15, 67 %) and median LVEF was 48 %.
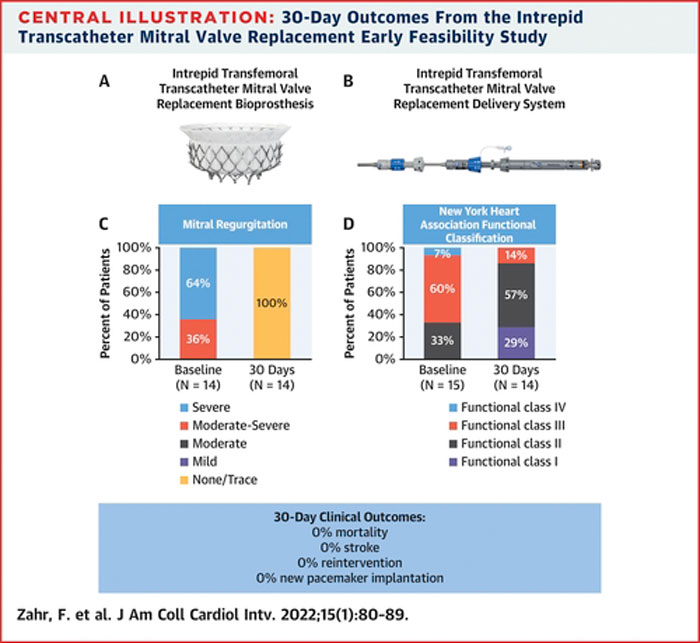
Acute procedural success (defined as successful surgical vascular access, valve delivery, system retrieval) was obtained in 14 patients. One conversion to sternotomy was required due to atrial valve migration deemed related to the presence of rheumatic changes of the subvalvular apparatus hindering adequate expansion and engagement of the prosthesis.
Median procedure time was 159 minutes, with a median of 46 minutes from delivery catheter insertion to removal.
In 11 patients (73 %) atrial septal defect closure was deemed necessary during index procedure.
Median length of in-hospital stay was 5 days, 2 patients had evidence of moderate LVOT obstruction while none showed significant residual regurgitation or stenosis.
At follow up 7 (47 %) major (non-fatal) vascular complications (6 access site related) and 1 left ventricular pseudoaneurysm (no further details provided) were recorded, while no deaths, strokes, re-interventions, or permanent PM implants were observed.
Source: JACC - Cardiovascular Interventions
Critical reading and the relevance for clinical practice
Present data, together with the results of the Evoque and Sapien M3 systems (Webb et al. J Am Coll Cardiol Intv. 2020;13: 2418–2426 and J Am Coll Cardiol. 2019;73: 1239–1246) represent a further step forward to the development of a class of devices that will allow to safely and effectively perform TMVR in selected patients.
The present report confirms the feasibility of trans-femoral TMVR. Short-term mortality reported in this small series is notably lower as compared to that observed among different trans-apical TMVR systems, including the Intrepid experience (14 % at 30-day in an earlier report by Bapat et al. J Am Coll Cardiol. 2018;71: 12–21). This confirms the well-known concept reducing invasiveness results in optimization of outcomes and that LV apical manipulation comes at price of increased short and long-term adverse events, thus supporting the efforts to develop a percutaneous approach for TMVR. Length of in-hospital stay is comparable to that of current TEER procedures.
Nonetheless, some crucial points need to be addressed to allow a wider implementation of this technique:
As evident from this report, high rates of procedural events including major vascular complications (related to the need of large-bore delivery systems), device embolization, and LVOT obstruction are still of relatively common occurrence in highly selected patients undergoing extensively well-planned procedures in high-volume centers.
In addition, as already occurred with TEER RCTs, enrollment of patients with predominant primary MR creates an “ideal virtual milieu” that will not be reflected after its implementation in clinical practice (with a known predominance of secondary MR as evident in clinical experience and according to registry derived data).
Finally, while in patients with secondary mitral regurgitation and impaired LVEF, the dynamic reduction of mitral regurgitation to a clinically tolerable level has been the target of TEER, the hemodynamic consequences deriving from a complete abolition of MR after TMVR might potentially represent a double edge sword, requiring further investigations in larger series.