Acute kidney injury following tricuspid transcatheter edge-to-edge repair
Selected in JACC: Cardiovascular Interventions by A. Mangieri , A. Sticchi
Although tricuspid regurgitation has for a long time been “the forgotten valve”, recently there has been a rising awareness of the disease with several important studies investigating its pathophysiology and treatment.
References
Authors
Tetsu Tanaka, Refik Kavsur, Atsushi Sugiura, Jean Marc Haurand, Natalia Galka, Can Öztürk, Johanna Vogelhuber, Marc Ulrich Becher, Marcel Weber, Ralf Westenfeld, Sebastian Zimmer, Malte Kelm, Georg Nickenig, Patrick Horn, Christian Zachoval
Reference
J Am Coll Cardiol Intv. 2022 Oct, 15 (19) 1936–1945
Published
15 October 2022
Link
https://www.jacc.org/doi/10.1016/j.jcin.2022.07.018Reviewers
Our Comment
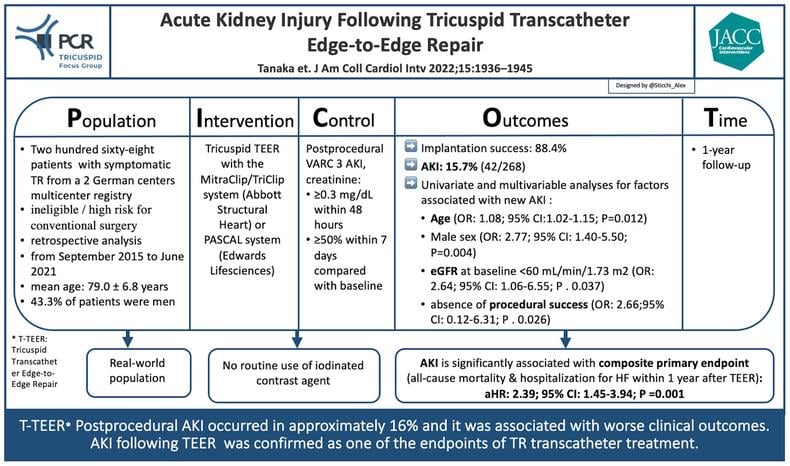
PICOT analysis of AKI and T-TEER - Courtesy of Alex Sticchi @Sticchi_Alex, Source: PCRonline.com
Why this study – the rationale/objective?
Tricuspid regurgitation (TR) has been for a long time “the forgotten valve” but recently, there has been a new rising awareness of the disease with several important studies that are investigating its pathophysiology and treatment [1].
TR is a common disease in the elderly population, and its presence and severity significantly impact long-term survival and hospitalization [2].
Transcatheter tricuspid therapy showed to be effective in improving symptoms and clinical outcomes. Still, we are investigating the prognostic impact and predictors for patients’ selection criteria to maximize the benefit of the intervention [3].
Acute kidney injury (AKI) was recognized as one of the most important predictors and selection criteria for valvular intervention with a significant impact on mortality and morbidity [4,5].
Recently, AKI has been associated with 30-day mortality after Transcatheter Edge-to-Edge repair (TEER), Odds ratio (OR): 8.06; 95% CI: 3.20, 20.30, p < 0.01; I2 = 18.4%) and all-cause mortality over a mean follow-up time of 30 months (Hazard ratio (HR): 2.48; 95% CI: 1.89, 3.24, p < 0.01; I2 = 23.7%) [6]. This renal impairment could be the result of both comorbidities and hemodynamic abnormalities, and it is independent of the use of contrast medium during the TEER procedure [6].
Tanaka et al. in the last issue of JACC Cardiovascular Intervention, investigated the prognostic impact of postprocedural AKI following TEER to treat TR [7]. In this study, the authors explored the differences between patients with and without new onset of AKI in terms of baseline characteristics, echocardiographic assessments, peri-procedural findings, and outcomes. They found a significant rate of post-procedural AKI in Tricuspid TEER (TTEER), and it was associated with worse clinical outcomes.
How was it executed – the methodology?
The study retrospectively analyzed data from a multicenter registry which included patients undergoing Tricuspid transcatheter intervention at 2 German high-volume centers (University Hospital Bonn and University Hospital Düsseldorf) from September 2015 to June 2021. Patients in end-stage renal therapy were not included. AKI was defined according to Valve Academic Research Consortium 3, consisting of a serum creatinine increase of ≥ 0.3 mg/dL within 48 hours compared with baseline or an increase in serum creatinine to ≥ 1.5 times baseline within 7 days [8]. The population was divided in patients with and without post-TEER AKI. The authors compared the two groups and assessed the study's Primary Endpoint (PE) represented by a composite outcome including all-cause mortality and hospitalization for heart failure at the 1-year follow-up after TEER.
What is the main result?
In the study of Tanaka et al., post-TTEER AKI occurred in 15.7% (42/268 patients) during a median hospitalization length of 8 days (IQR: 6-10 days). 80.9% of them are in AKI stage I (increase in serum creatinine of ≥ 0.3 mg/dL within 48 hours or of 1.50 to 1.99 times within 7 days), 16.7% in stage II (increase of 2.00 to 2.99 times compared with baseline) and 2.4% in stage III (increase of ≥3.00 times or serum creatinine >4.0 mg/dL with an acute increase of at least 0.5mg/dL or the need for renal replacement therapy).
Procedural success was achieved in the (88.4%) and it was associated with higher incidence of postprocedural AKI compared with those with procedural success (29.0% vs 13.9%; P = 0.038; (OR: 2.66; 95% CI: 0.12-6.31; P = 0.026).
Moreover, similarly to procedural success, age (OR: 1.08; 95% CI: 1.02-1.15; P = 0.012), male sex (OR: 2.77; 95% CI: 1.40- 5.50; P = 0.004), eGFR at baseline <60 mL/min/1.73 m2 (OR: 2.64; 95% CI: 1.06-6.55; P = 0.037) were consistently associated with AKI incidence in the multivariable model.
In a smaller number of patients (212/268), the pre-procedural right heart catheterization showed the association of right atrial pressure (OR: 1.07; 95% CI: 1.01-1.12; P = 0.014) and systolic pulmonary artery pressure (OR: 1.04; 95% CI: 1.01-1.06; P = 0.002) with the incidence of postprocedural AKI.
The Primary Endpoint was significantly higher in patients with AKI (66.2% versus [vs] 28.1% of the group without post-procedural AKI; P < 0.001). Furthermore, in-hospital mortality (9.5% vs 0.9%; P = 0.006), major bleeding (or life-threatening bleeding) events (9.5% vs 2.2%, P = 0.037) and length of hospitalization (8 days [6-13] vs 7 [6-10], P = 0.041) were higher in patient with AKI compared with patients without AKI.
Critical reading and relevance for clinical practice
As previously proved in the Mitral scenario, the interesting study of Tanaka et al. confirmed the not negligible clinical impact of TEER in terms of post-procedural AKI in the Tricuspid treatment.
This adverse effect is independent of the use of iodinated contrast medium.
Moreover, AKI is associated with all-cause mortality or hospitalization for heart failure at the 1-year follow-up and in-hospital mortality. This last evidence is also supported by the recent TRISCORE that includes a weighted role for renal function.
We must recognize some limitations of the study, first the retrospective design, and secondarily the lack of a predetermined protocol for postprocedural assessments of creatinine level. However, the value of the study is unmistakable because it highlighted the AKI issue and its prognostic impact in a procedure without iodinated contrast medium.
Besides the several AKI predictors reported in the study, the clinical significance of the procedural success should push us to carefully consider TEER when the initial chances of being effective are poor.
Further dedicated studies will clarify the TEER impact on AKI and the procedure's adverse predictors to maximize the balance between safety and efficacy.
References:
- Praz F, Muraru D, Kreidel F, Lurz P, Hahn RT, Delgado V, Senni M, Bardeleben RS von, Nickenig G, Hausleiter J, Mangieri A, Zamorano JL, Prendergast BP, Maisano F. Transcatheter treatment for tricuspid valve disease. EuroIntervention. 19AD;17:791–808.
- Topilsky Y, Nkomo VT, Vatury O, Michelena HI, Letourneau T, Suri RM, Pislaru S, Park S, Mahoney DW, Biner S, Enriquez-Sarano M. Clinical Outcome of Isolated Tricuspid Regurgitation. JACC Cardiovasc Imaging. 2014;7:1185–94.
- Brener MI, Lurz P, Hausleiter J, Rodés-Cabau J, Fam N, Kodali SK, Rommel KP, Muntané-Carol G, Gavazzoni M, Nazif TM, Pozzoli A, Alessandrini H, Latib A, Biasco L, Braun D, Brochet E, Denti P, Lubos E, Ludwig S, Kalbacher D, Estevez-Loureiro R, Connelly KA, Frerker C, Ho EC, Juliard JM, Harr C, Monivas V, Nickenig G, Pedrazzini G, Philippon F, Praz F, Puri R, Schofer J, Sievert H, Tang GHL, Andreas M, Thiele H, Unterhuber M, Himbert D, Alcázar MU, Von Bardeleben RS, Windecker S, Wild MG, Maisano F, Leon MB, Taramasso M, Hahn RT. Right Ventricular-Pulmonary Arterial Coupling and Afterload Reserve in Patients Undergoing Transcatheter Tricuspid Valve Repair. J Am Coll Cardiol. 2022;79:448–61.
- Dreyfus J, Audureau E, Bohbot Y, Coisne A, Lavie-Badie Y, Bouchery M, Flagiello M, Bazire B, Eggenspieler F, Viau F, Riant E, Mbaki Y, Eyharts D, Senage T, Modine T, Nicol M, Doguet F, Nguyen V, Le Tourneau T, Tribouilloy C, Donal E, Tomasi J, Habib G, Selton-Suty C, Raffoul R, Iung B, Obadia JF, Messika-Zeitoun D. TRI-SCORE: a new risk score for in-hospital mortality prediction after isolated tricuspid valve surgery. Eur Heart J. 2022;43:654–62.
- Julien HM, Stebbins A, Vemulapalli S, Nathan AS, Eneanya ND, Groeneveld P, Fiorilli PN, Herrmann HC, Szeto WY, Desai ND, Anwaruddin S, Vora A, Shah B, Ng VG, Kumbhani DJ, Giri J. Incidence, Predictors, and Outcomes of Acute Kidney Injury in Patients Undergoing Transcatheter Aortic Valve Replacement: Insights From the Society of Thoracic Surgeons/American College of Cardiology National Cardiovascular Data Registry-Transcatheter Valve Therapy Registry. Circ Cardiovasc Interv. 2021;14:E010032.
- Doulamis IP, Tzani A, Kampaktsis PN, Kaneko T, Tang GHL. Acute Kidney Injury Following Transcatheter Edge-to-Edge Mitral Valve Repair: A Systematic Review and Meta-Analysis. Cardiovasc Revascularization Med. 2022;38:29–35.
- Tetsu Tanaka M, Refik Kavsur M, Atsushi Sugiura MP, Jean Marc Haurand M, Natalia Galka M, Can Öztürk M, Johanna Vogelhuber M, Marc Ulrich Becher M, Marcel Weber M, Ralf Westenfeld M, Sebastian Zimmer M, Malte Kelm M, Georg Nickenig M, Patrick Horn M, Christian Zachoval M. Acute Kidney Injury Following Tricuspid Transcatheter Edge-to-Edge Repair. Cardiovasc Interv. 2022.
- Généreux P, Piazza N, Alu MC, Nazif T, Hahn RT, Pibarot P, Bax JJ, Leipsic JA, Blanke P, Blackstone EH, Finn MT, Kapadia S, Linke A, Mack MJ, Makkar R, Mehran R, Popma JJ, Reardon M, Rodes-Cabau J, Van Mieghem NM, Webb JG, Cohen DJ, Leon MB. Valve Academic Research Consortium 3: updated endpoint definitions for aortic valve clinical research. Eur Heart J. 2021;42:1825–57.






No comments yet!