Pre-treatment with P2Y12 inhibitors in patients with chronic coronary syndrome undergoing percutaneous coronary intervention: a report from the Swedish Coronary Angiography and Angioplasty Registry
Selected in Circulation: Cardiovascular Interventions by K. De Silva , T. Suji
The optimal timing of the administration of P2Y12 inhibitors in patients with CCS undergoing ad-hoc PCI is unknown. This study aimed to provide observational data to clarify these quandaries.
References
Authors
Juliane Jurga, Karolina Elizabeth Szummer, Christian Lewinter, Linda Mellbin, Matthias Götberg, Sammy Zwackman, Johan Nilsson, Sebastian Völz, David Erlinge, Jonas Persson, Elmir Omerovic, Tomas Jernberg and Dimitrios Venetsanos
Reference
10.1161/CIRCINTERVENTIONS.121.010849
Published
1 Oct 2021
Link
Read the abstract
Reviewers
Our Comment
Why this study – the rationale/objective?
This was a retrospective, multicentre study (74 hospitals) using the Swedish Coronary Angiography and Angioplasty Registry to analyze 26,814 chronic coronary syndrome (CCS) patients undergoing elective coronary angiography (CA) and ad-hoc percutaneous coronary intervention (PCI) between 2006-2017.
The optimal timing of the administration of P2Y12 inhibitors in patients with CCS undergoing ad-hoc PCI is unknown. It is also unclear whether ticagrelor/ prasugrel or clopidogrel results in the best outcomes when administered in-cathlab.
This study aimed to provide observational data to clarify these quandaries.
How was it executed? - the methodology
Timing of administration of a P2Y12 inhibitor - clopidogrel, ticagrelor, or prasugrel - was divided into two groups: pre-treatment and in-cathlab.
Pre-treatment was defined as a P2Y12 inhibitor administered within 24 hours before CA, outside the catheterization laboratory. This included 18,577 patients, 69 % of the total. Of the pre-treatment group, 9 % received ticagrelor, 0.5 % prasugrel and 90.5 % received clopidogrel.
In-cathlab was defined as a P2Y12 inhibitor administered inside the cathlab. This included 8,237 patients, 31 % of the total. Of the in-cathlab group, 34.6 % received ticagrelor, 6.5 % received prasugrel, and 59 % received clopidogrel. There is no clarity provided about whether the in-cathlab group had this immediately prior to, or immediately following their PCI procedure.
Patients were followed up until the time of an adverse event, or for up to 30 days following the CA or PCI. Adverse events were split into primary (includes all-cause mortality, myocardial infarction, stroke, or any bleeding complication) and secondary events (major adverse cardiac and cerebrovascular events including all causes of mortality, MI, or stroke, the individual components of net adverse clinical events and in-hospital bleeding).
What is the main result?
The baseline characteristics of the in-cathlab group compared to the pre-treatment group had reduced incidence of diabetes (24.7 % versus 26.5 % p = <0.01), previous MI (27.6 % versus 32.6 % p = <0.01), and previous PCI (25.4 % versus 27.6 % p = <0.01) and were more likely to receive ticagrelor/prasugrel. Procedurally, they were less likely to have radial access (69.2 % versus 67.5 % p = 0.01).
The pre-treatment group experienced no reduction in ischaemic complications but had an increased risk of in-hospital bleeding 2.1 % versus 1.9 %, with an adjusted odds ratio with 95 % confidence interval of 0.70 (0.51-0.96). There was a net adverse risk of 5.1 % in pre-treatment compared to 4.2 % for in-cathlab treatment patients, with an adjusted hazard ratio with 95 % confidence interval of 0.79 (0.63-0.99).
Ticagrelor/ prasugrel versus clopidogrel was only analyzed in the in-cathlab group (due to the low numbers of patients given these P2Y12 inhibitors in the pre-treatment group). Ticagrelor/ prasugrel treated patients were more likely to have hypertension (77.2 % versus 72.2 % p = <0.01) and less likely to have a previous MI (25.6 % versus 29.1 % p = <0.01) than clopidogrel-treated patients. Ticagrelor/ prasugrel treated patients had more frequent use of the radial access (80.2 % versus 61.6 % p < 0.01) and with a trend toward receiving less intra-procedural glycoprotein IIb/IIIa receptor inhibitor (0.3 % versus 3.1 % p = 0.07).
Ticagrelor/ prasugrel given in-cathlab did not reduce ischaemic complication risk but increased the risk of bleeding compared to clopidogrel. There was a net adverse risk of 5.4 % in prasugrel/ ticagrelor administered patients compared to 3.4 % in those administered with clopidogrel with an adjusted hazard ratio of 95 % confidence interval of 1.66 (1.12-2.48). The absolute in-hospital (2.9 % versus 1.2 %, adjusted HR 2.14 (1.34-3.42)) and 30-day (3.4 % versus 1.6 %, adjusted HR 2.24 (1.29-3.90)) for all bleeding events were greater than two-fold higher and exactly two-fold greater for major in-hospital bleeding (1.0 versus 0.5, adjusted HR 2.54 (1.42-4.56)).
In both comparisons, there was no significant difference in risk of death, myocardial infarction, or stroke, and no statistical difference in sex and mean age baseline characteristics.
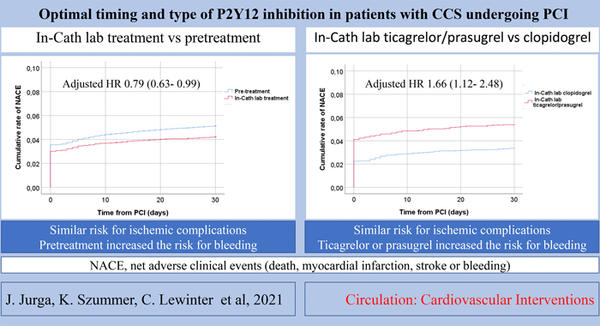
Summary of net adverse clinical events.
Source: American Heart Association Journals
Critical reading and the relevance for clinical practice?
The findings of this study suggest that there is no difference in major adverse cardiac or cerebrovascular events in patients undergoing PCI for chronic coronary syndrome, in those pre-treated with a P2Y12 inhibitor compared to those who have this administered in the cath lab. Furthermore, this study suggests that those who have pre-treatment of P2Y12 inhibitors have an increased risk of bleeding.
Whilst these data are informative and may provide guidance about the necessity of pre-treatment of P2Y12 inhibitor therapy in the setting of chronic coronary syndrome, there are several important limitations that mean the external generalisability of this study may be reduced.
Firstly, in the in-cathlab group, it has not been specified whether the P2Y12 inhibitor is given before or immediately after the procedure. In those that have anti-platelet therapy administered immediately prior to their PCI procedure, they may be getting partial pre-treatment, as the anti-platelets are being absorbed prior to, or during, the PCI procedure. This is distinct to those who undergo a PCI procedure and then have the P2Y12 therapy after the procedure. This study does not specifically clarify whether this second group, who is completely naïve to P2Y12 inhibitor therapy, suffers adverse outcomes due to the delay in administering the anti-platelet therapy. However, the absence of any signal of benefit in the pre-treatment group in this study would suggest that this is unlikely to be relevant and the exact timing of P2Y12 administration may not important in the setting of chronic coronary syndrome.
Secondly, the follow-up time point is limited to 30-days. Whilst early stent thrombosis events are likely to have been captured, a longer follow-up, out to 12 months, would have provided more robust and definitive comparative outcomes between the two groups.
Thirdly, there were numerous and significant differences in the baseline and procedural characteristics, between the pre-treatment and in-cathlab groups. The pre-treatment group had a higher incidence of hypertension, hypercholesterolaemia, diabetes, prior MI, prior PCI, and prior stroke with less radial access utilization compared to the in-cathlab group. This more co-morbid group had higher rates of in-hospital bleeding, which is likely to be due to a combination of co-morbidity and the reduced radial access use. Despite these marked differences, this did not translate to a difference in overall all-cause mortality or cardiovascular outcomes (MI and stroke) between the two groups at 30-days. Nonetheless, any benefit provided by pre-treatment with P2Y12 inhibitor therapy may have been masked by these differences and warrants further investigation in a more matched cohort or a randomized control trial.
In summary, this study suggests in-cathlab treatment with P2Y12 inhibitors in those undergoing elective PCI for chronic coronary syndrome may be sufficient and that this strategy may lead to reduced bleeding with no difference in ischaemic events. However, due to the observational nature of this registry and the significant variation between the two groups analyzed further ratification with a randomized trial would be optimal.
References
- Jernberg T, Attebring MF, Hambraeus K, Ivert T, James S, Jeppsson A, Lagerqvist B, Lindahl B, Stenestrand U, Wallentin L. The Swedish Web-system for enhancement and development of evidence-based care in heart disease evaluated according to recommended therapies (SWEDEHEART). Heart. 2010 Oct;96(20):1617-21.
- Dworeck C, Redfors B, Völz S, et al. Radial artery access is associated with lower mortality in patients undergoing primary PCI: a report from the SWEDEHEART registry. Eur Heart J Acute Cardiovasc Care. 2020;9(4):323-332.






No comments yet!