Transcatheter aortic valve replacement with self-expanding ACURATE neo2
Selected in JACC: Cardiovascular Interventions by M. Pighi , M. Lunardi
This study investigated the short-term safety and efficacy of the new valve ACURATE neo2 in comparison to the first neo model in a real-world population.
References
Authors
Buono A, Gorla R, Ielasi A, Costa G, Cozzi O, Ancona M, Soriano F, De Carlo M, Ferrara E, Giannini F, Massussi M, Fovino LN, Pero G, Bettari L, Acerbi E, Messina A, Sgroi C, Pellicano M, Sun J, Gallo F, Franchina AG, Bruno F, Nerla R, Saccocci M, Villa E, D'Ascenzo F, Conrotto F, Cuccia C, Tarantini G, Fiorina C, Castriota F, Poli A, Petronio AS, Oreglia J, Montorfano M, Regazzoli D, Reimers B, Tamburino C, Tespili M, Bedogni F, Barbanti M, Maffeo D; ITAL-neo Investigators.
Reference
JACC Cardiovasc Interv. 2022 Jun 13;15(11):1101-1110. doi: 10.1016/j.jcin.2022.02.027. Epub 2022 May 17. PMID: 35595675.
Published
15 June 2022
Link
Read the abstractReviewers
Our Comment
Why this study – the rationale/objective?:
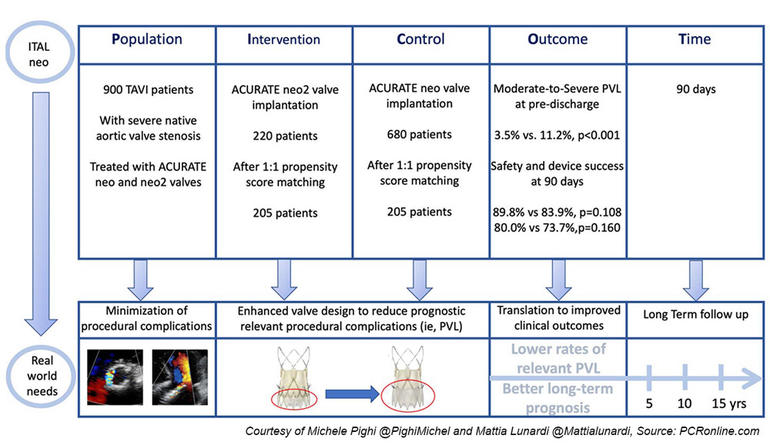
PICOT analysis of the ITAL neo Registry by M. Pighi & M. Lunardi
Transcatheter aortic valve implantation (TAVI) is the preferred treatment for symptomatic aortic valve stenosis in patients older than 75 years, regardless of the surgical risk. As such, the number of patients profiting from this treatment is expected to grow even more.
Considering that, minimizing procedural complications leading to adverse outcomes is essential. Among these, residual paravalvular leak (PVL) is one of the most frequent concerns after TAVI, as proven to be related to a worse prognosis. The prosthesis design is among the factors responsible for such a complication.
The supra-annular self-expandable ACURATE neo valve prosthesis (Boston Scientific) counted higher rates of moderate-to-severe PVL than other competitors, limiting the use of this valve to selected patients. To overcome this issue, a new iteration to the first valve model (ACURATE neo2) has been made, lengthening the outer pericardial skirt to the upper crown level, aiming at reducing the severity of PVL.
Pending the results of the dedicated ongoing randomized trial ACURATE IDE, this study investigated the short-term safety and efficacy of the new valve ACURATE neo2 in comparison to the first neo model in a real-world population.
How was it executed? - the methodology:
ITAL-neo was an observational, retrospective, multicenter, investigator-initiated registry.
Patients deemed suitable for TAVI with ACURATE neo and neo2 valves through trans-femoral or trans-subclavian routes were included in the study. Both bicuspid and tricuspid anatomies were included.
To account for the non-randomized design, a propensity score matching was used to adjust for baseline confounding variables between neo and neo2 groups.
The primary endpoint was the rate of moderate-to-severe PVL at discharge as assessed by echocardiography.
Secondary endpoints included the post-procedural, technical success, and the composite device success and safety at 90 days, according to VARC-3 criteria.
What is the main result?
- The enrolled population included 900 patients, of whom 680 received the first neo valve, whereas 220 the upgraded neo2 model.
- After the propensity score, 205 patients per group were matched, resulting in a total population of 410 patients.
- The primary endpoint occurred in 11.2 % vs 3.5 % of patients receiving the neo and the neo2 valve, respectively (p < 0.001).
- Among the secondary endpoints, the post-procedural and technical success was comparable between the two groups (95.1 % vs 97.6 %, p = 0.293). Similarly, no difference was reported for the 90-day device success and safety endpoints (83.9 % vs 89.8 %; p = 0.108 and 73.7 % vs 80.0 %; p = 0.160, respectively).
Critical reading and the relevance for clinical practice:
The first model of the ACURATE neo valve is a self-expanding device in a supra-annular position that is implanted in a top-down two-step release mechanism. This unique deployment mechanism, supported by the three stabilization arches, minimizes peri-procedural outflow obstruction and allows for stable positioning without rapid ventricular stimulation. This specific release, together with a very flexible delivery system, makes this valve ideal also in horizontal aortas.
The device presents additional promising features. Among these, the short stent body and the open-cell design of the upper crown reassure about the risk of coronary artery obstruction and coronary re-engagement.
Yet, the limited radial force of the device represents an advantage in terms of electrical disturbances and, therefore, pacemaker necessity after the valve implantation.
Unfortunately, such potential benefits have been thwarted by higher rates of procedural complications, including the tripled rates of moderate-to-severe PVL, leading to a double risk of valve-related dysfunction and, therefore, worse prognosis compared to the balloon-expandable SAPIEN 3 valve1.
In light of such limitations, the upgraded ACURATE neo2 model has been recently launched, maintaining the original design but enhanced with a taller sealing outer pericardial skirt in order to shoot down the rates of relevant PVL.
Similarly, to previous smaller series2, the present study offers preliminary results about the efficacy and safety of the neo2 device in a real-world population.
The implemented new features of the neo2 model seem to give hope back to the ACURATE valve, significantly reducing the occurrence of residual PVL in this study, amounting to 3.5 %, three-time less than the first version. Such rate is aligned with the ones of the most popular devices on the market, with SAPIEN 3 and EVOLUT PRO valves presenting a 2.8 % and 5.7 % incidence, respectively, in head-to-head comparisons with ACURATE neo1,3.
Noteworthy, the worse outcomes of ACURATE neo presented in the SCOPE trial1 - namely valve dysfunction, acute kidney injury (AKI), and need for multiple valves- appear all deriving from the higher incidence of significant PVL.
Indeed, as confirmed in the ITAL-neo study, post-dilatation was much more frequent after the first model implantation, requiring additional use of contrast, also leading to higher rates of AKI.
Consequently, minimizing the PVL after the valve implantation might seriously represent the game-changer for this device.
If these early findings are confirmed in the dedicated randomized trial ACURATE IDE [NCT03735667], comparing ACURATE neo2 with CoreValve and SAPIEN 3 system, we can expect a rapid spread of this technology among TAVI patients, considering the peculiar benefits offered by this device.
References
- Lanz J, Kim WK, Walther T, et al. Safety and efficacy of a self-expanding versus a balloon-expandable bioprosthesis for transcatheter aortic valve replacement in patients with symptomatic severe aortic stenosis: a randomised non-inferiority trial. Lancet. 11 02 2019;394(10209):1619-1628. doi:10.1016/S0140-6736(19)32220-2
- Rück A, Kim WK, Kawashima H, et al. Paravalvular Aortic Regurgitation Severity Assessed by Quantitative Aortography: ACURATE. J Clin Med. Oct 09 2021;10(20) doi:10.3390/jcm10204627
- Pagnesi M, Kim WK, Conradi L, et al. Transcatheter Aortic Valve Replacement With Next-Generation Self-Expanding Devices: A Multicenter, Retrospective, Propensity-Matched Comparison of Evolut PRO Versus Acurate neo Transcatheter Heart Valves. JACC Cardiovasc Interv. 03 11 2019;12(5):433-443. doi:10.1016/j.jcin.2018.11.036







No comments yet!