26 Jan 2023
2-year outcomes of transcatheter mitral valve replacement in patients with annular calcification, rings, and bioprostheses
Selected in Journal of the American College of Cardiology by E. Zancanaro
The aim of this study is to present the TMVR results at 2 years from the MITRAL population.
References
Authors
Mackram F. Eleid, Dee Dee Wang, Amit Pursnani, Susheel K. Kodali, Issac George, Igor Palacios, Hyde Russell, Raj R. Makkar, Saibal Kar, Lowell F. Satler, Vivek Rajagopal, George Dangas, Gilbert H.L. Tang, James M. McCabe, Brian K. Whisenant, Kenith Fang, Tatiana Kaptzan, Bradley Lewis, Pamela Douglas, Rebecca Hahn, Jeremy Thaden, Jae K. Oh, Martin Leon, William O'Neill, Charanjit S. Rihal, and Mayra E. Guerrero
Reference
J Am Coll Cardiol. 2022 Dec, 80 (23) 2171–2183
Published
December 2022
Link
Read the abstract
Reviewer
My Comment
Why this study – the rationale/objective?
Transcatheter mitral valve replacement (TMVR) has become increasingly important in recent decades for patients at high surgical risk.
The first randomized controlled trial MITRAL (Mitral Implantation of Transcatheter Valves) showed excellent results at 1 year in terms of survival. Results beyond 1 year have to be seen.
The aim of this study is to present the TMVR results at 2 years from the MITRAL population.
How was it executed? - the methodology
The present trial, an early feasibility study, is a physician-initiated, prospective, multicentered clinical trial designed to evaluate the safety and feasibility of TMVR using SAPIEN XT and SAPIEN 3 valves (Edwards Lifesciences) with 3 treatment arms:
- native MV disease with MAC
- failed MV annuloplasty ring
- failed surgical bioprosthesis.
Principal inclusion criteria included patients with severe mitral stenosis, or severe MR, and New York Heart Association (NYHA) functional class II or more symptoms, who were at high surgical risk after Heart Team evaluation.
Three important types of procedures have been discussed:
- valve-in-valve (ViV)
- valve-in-ring (ViR)
- and valve-in-mitral annular calcification (ViMAC).
Study endpoints
- Mitral valve status: Absence of MR grade 2+ or higher and mean MV gradient >10 mm Hg
- Survival: All-cause mortality at 2 years
- Quality-of-life endpoints: NYHA functional class, Kansas City Cardiomyopathy Questionnaire (KCCQ) scores, and 6-minute walk distance at 2 years
What is the main result?
The results have been subdivided into 3 arms: ViV, ViR and ViMAC
- ViV
- Mortality --> occurred in 2 of 30 patients (6.7 %), of which 1 of 30 (3.3 %) was cardiovascular in etiology. The incidence of stroke was 6.7 % at 2 years. There were no cases of valve thrombosis and endocarditis. No hospitalization for heart failure.
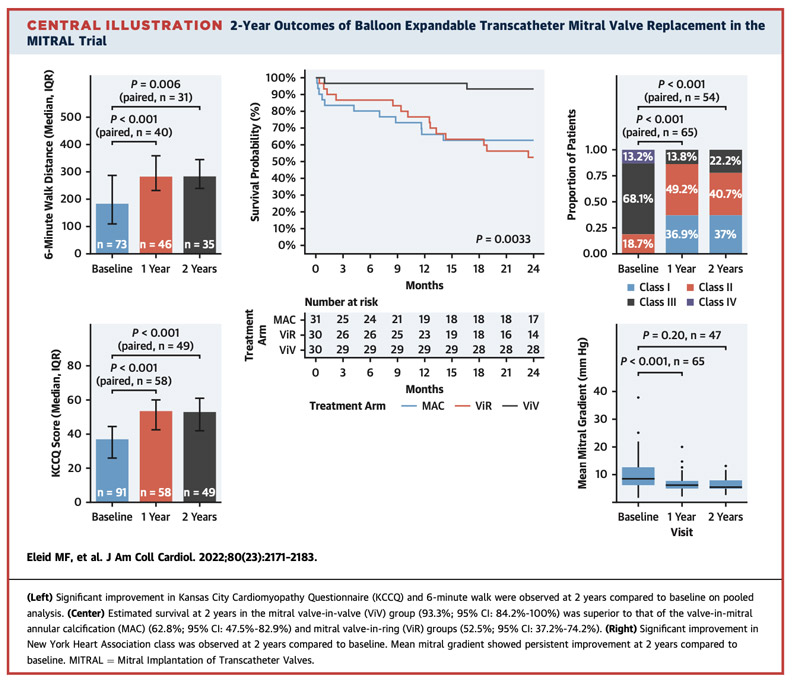
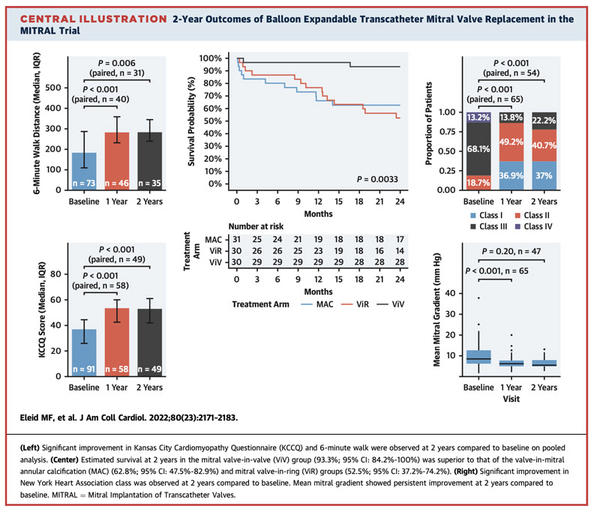
- Quality of life at 2 years --> The NYHA functional class shows improvement at 2 years compared to baseline, with 85 % of patients in NYHA functional class I or II. The KCCQ scores show improvement at 2 years compared to baseline (2 years: 55 [IQR: 48.75-62]).
- ViR
- Mortality --> occurred in 13 of 30 patients (43.3 %), of which 5 of 30 (17 %) were cardiovascular in etiology. Between 1 and 2 years of follow-up, 6 deaths occurred, 3 (50 %) of which were cardiovascular and the remainder were non-cardiovascular. Of the 3 cardiovascular deaths, 1 was determined to be device-related; 1 was sudden death in a patient who had developed LVOT obstruction post-TMVR. The incidence of stroke was 3.3 % at 2 years. There were no cases of prosthetic valve thrombosis and endocarditis. Rehospitalization for heart failure occurred in 27 % of patients.
- Quality of life -->The NYHA functional class shows improvement at 2 years compared to baseline, with 75 % of patients in NYHA functional class I or II. The KCCQ scores showed improvement at 2 years on paired analysis compared to baseline (2 years: 49 [IQR: 39-59.5]).
- ViMAC
- Mortality --> occurred in 11 of 28 patients (39.3 %) of which 6 of 28 (21.4 %) were cardiovascular in etiology. The incidence of stroke was 10.7 % at 2 years. Between 1 and 2 years, there was 1 additional case of prosthetic valve thrombosis making the total 2-year incidence 7.1 % (2 of 28 patients). No cases of endocarditis were observed at 2 years. No hospitalizations for heart failure occurred between 1 and 2 years of follow-up.
- Quality of life --> NYHA functional class continued to show improvement at 2 years compared to baseline, with 66.7 % of patients in NYHA class I or II. KCCQ (median: baseline: 34 [IQR: 26-44]; 1 year: 53 [IQR: 47-64], 2 years: 51 [IQR: 42.25-56.75]).
Source: Journal of the American College of Cardiology

Source: Journal of the American College of Cardiology
Critical reading and the relevance for clinical practice
The present study shows the TMVR results in the case of ViR, ViV, and ViMAC at 2 years follow-up.
The results of this study demonstrated:
- Quality of life measures, including NYHA functional class and KCCQ score improvements in all 3 arms
- Echocardiographic analysis showed stable TMVR prosthetic valve function at 2 years with low transvalvular gradients and low rates of MR
- Very low rates of transcatheter heart valve thrombosis and endocarditis were observed at 2 years
- Survival at 2 years was excellent in the MViV group, whereas 2-year survival was lower in the MViR and ViMAC groups, with higher mortality noted in the MViR group between 1 and 2 years.
Three considerations have to be made:
- TMVR is a valid option in case of high surgical risk patients; it represents a procedure that can embrace different kinds of patients with different mitral valve settings, from the native MR to the mitral valve prosthesis dysfunction. It also showed a valid improvement in clinical condition and quality of life for high-risk profile patients.
- Still, there is a difference in results comparing the different TMVR interventions; this difference has to be addressed in the future.
- What remains to be addressed is for sure the faith of the not-eligible patients and what has to be done in this category of patients.
In conclusion, the use of balloon-expandable aortic transcatheter heart valves for selected patients with severe MAC, failed annuloplasty ring, and bioprosthetic MV dysfunction is associated with sustained improvements in quality of life and heart failure symptoms at 2 years of follow-up. Transcatheter heart valve prosthesis function remained stable between year 1 and year 2. The 2-year survival estimates were greater in the MViV than in the MViR and ViMAC groups.