19 Mar 2023
Management of coronary artery disease in patients undergoing transcatheter aortic valve implantation
Selected in EuroIntervention Journal by A. N. Calik
This position paper concentrated on the diagnosis and management of severe CAD in TAVI candidates, THV commissural alignment, coronary re-access after TAVI, and redo-TAVI.
References
Authors
Giuseppe Tarantini, Gilbert Tang, Luca Nai Fovino, Daniel Blackman, Nicolas M. Van Mieghem, Won-Keun Kim, Nicole Karam, Pedro Carrilho-Ferreira, Stephane Fournier, Jerzy Pręgowski, Chiara Fraccaro, Flavien Vincent, Rui Campante Teles, Darren Mylotte, Ivan Wong, Gintautas Bieliauskas, Martin Czerny, Nikolaos Bonaros, Alessandro Parolari, Darius Dudek, Didier Tchétché, Hélène Eltchaninoff, Ole de Backer, Giulio Stefanini, Lars Sondergaard
Reference
DOI: 10.4244/EIJ-D-22-00958
Published
22 February 2023
Link
Read the abstract
Reviewer
My Comment
This is a clinical consensus statement from the European Association of Percutaneous Cardiovascular Interventions, in collaboration with the ESC Working Group on Cardiovascular Surgery
Introduction
Severe coronary artery disease (CAD) is a common finding in patients with severe aortic stenosis receiving transcatheter aortic valve implantation (TAVI), and the management of these two disorders becomes more crucial as the TAVI procedure has extended to younger and lower-risk individuals (Figure 1).
The preprocedural diagnostic examination and criteria for treating substantial CAD in TAVI candidates are still up for debate despite TAVI having reached its maturity, as the recommended treatment for the majority of patients with tricuspid AS who are > 75 years old.
This position paper intends to present a rational and practical approach to the management of CAD in patients with symptomatic severe AS undergoing TAVI. It is concentrated on the diagnosis and management of severe CAD in TAVI candidates, THV commissural alignment, coronary re-access after TAVI, and redo-TAVI.
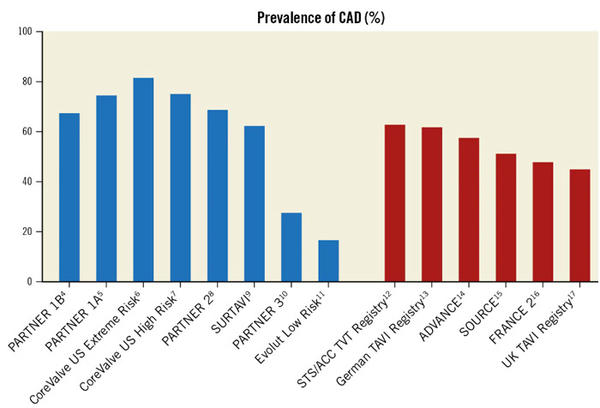
Figure 1. Prevalence of coronary artery disease (CAD) in patients treated with transcatheter aortic valve implantation as reported in randomised clinical trials (blue columns) and real-world registries (red columns). STS/ACC TVT: Society of Thoracic Surgeons/American College of Cardiology Transcatheter Valve Therapy.
Source: EuroIntervention Journal
Diagnostic evaluation of CAD in patients undergoing TAVI
Invasive Coronary Angiography
Invasive coronary angiography (ICA) remains the current standard of care in assessing CAD among patients undergoing TAVI and has the following advantages:
- Elderly patients scheduled for TAVI often have coronary arteries with a significant burden of calcium – limiting the diagnostic performance of coronary CT angiography (CTCA).
- Relevant guidelines recommend making decisions on coronary revascularisation based primarily on the angiographic degree of disease severity.
- In cases of intermediate CAD severity, ICA may allow immediate invasive physiological and imaging assessment of CAD, as well as the potential to proceed directly to PCI in the same procedure.
However, ICA does offer drawbacks, such as a higher risk of vascular complications, contrast nephropathy, more burden on the healthcare system, and a tendency to delay the treatment of AS. The majority of operators perform ICA prior to TAVI to reduce surgery time and contrast volume as well as to alert the heart team of the necessity for revascularization, which may affect the decision between SAVR+CABG and TAVI+PCI.
Invasive Coronary Physiology Assessment
There are conflicting results from the few observational studies and a lack of randomised trial data that address invasive physiological assessment in patients with severe AS. Hence, the use of the conventional thresholds of FFR ≤ 0.80 and iFR ≤ 0.89 is advisable.
Further study is required to determine the clinical utility of invasive coronary physiology in the setting of severe AS, especially to validate (non-) hyperaemic cut-offs to guide revascularization.
Non-Invasive CAD Assessment
Patients who have a low pre-test likelihood of having CAD and who are anticipated to have acceptable image quality may be candidates for CTCA. Given that the prevalence of significant CAD among elderly TAVI recipients approaches 50 %, including prior PCI in 20 %, and that significant coronary calcification is frequent, CTCA is unlikely to be of adequate diagnostic quality for the majority of pre-TAVI CAD assessments.
Management of CAD in patients undergoing TAVI
The optimal timing for performing PCI (before, concomitant, or following TAVI) in patients undergoing TAVI is still unknown, and the limited data is based solely on retrospective registry data. Each approach has benefits and drawbacks (Table 2).
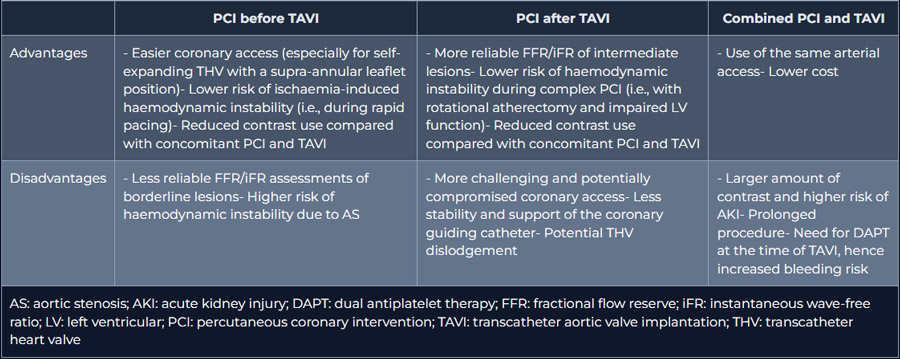
Table 2. Advantages and disadvantages of different PCI timing in patients undergoing TAVI.
Source: EuroIntervention Journal
It has been recommended to perform PCI, either as a staged or concomitant procedure, before TAVI due to the potential difficulties in restoring access to the coronary arteries after TAVI. Of note, cannulation of the coronary ostia may be more challenging, especially after the implantation of self-expanding THVs with a supra-annular leaflet position. Therefore, the patient's characteristics, such as the presence or absence of angina, the severity of the CAD, the location and complexity of the lesion, and the type of implanted THV, should be taken into consideration when making a decision on PCI in patients scheduled for TAVI. Although the available evidence is sparse, it generally discourages routine PCI before TAVI in asymptomatic lesions and recommends that when PCI is attempted, a separate staged procedure before TAVI is preferable.
The treatment of patients undergoing TAVI with co-existing CAD will be clarified by the findings of ongoing TAVI PCI (ClinicalTrials.gov: NCT04310046) and COMPLETE TAVR (ClinicalTrials.gov: NCT04634240) trials.
Antithrombotic Therapy Post-TAVI and PCI
As TAVI patients frequently have concomitant high bleeding risk factors, including age > 75 years, the balance between the benefit of reduced ischaemic events and the risk of bleeding remains the primary determinant in decision-making.
According to related guidelines, DAPT for 3 months should be followed by SAPT in patients with a high bleeding risk and no indication for OAC. When long-term OAC is indicated, triple therapy should be discontinued after one week and replaced with OAC+SAPT for a period of six months. Triple therapy should be avoided in cases of high or very high bleeding risk, and SAPT+OAC can be stopped after 1-3 months and replaced with OAC alone.
Coronary access and TAVI
According to large-scale TAVI registries, approximately 2 % of TAVI patients may require ICA and/or PCI within the first year after TAVI. Within 5 years of follow-up, ICA is performed in up to 16 % of TAVI patients, and approximately 5 % of these patients undergo PCI of one or more coronary lesions. Multiple factors influence the selection of THV, including the patient's individual anatomical suitability, availability, and operator experience.
However, given recent improvements in procedural outcomes, long-term considerations such as THV durability, redo-TAVI feasibility, conduction abnormalities, and the possibility of future coronary access have become important.
The THV design, particularly the stent frame height and leaflet position, can have an impact on future coronary access. Thus, familiarity with available THV devices is essential, even for interventional cardiologists who do not perform TAVI (Figures 3 & 4).
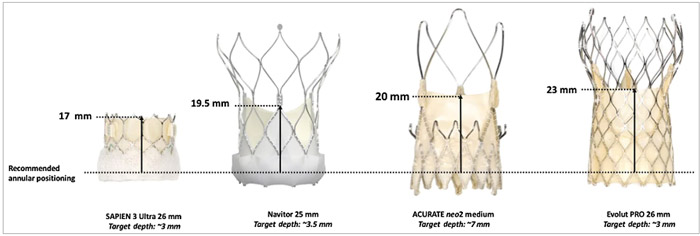
Figure 3. THV leaflet height with respect to recommended annular positioning. THV: transcatheter heart valve
Source: EuroIntervention Journal
![Figure 4. Coronary access according to valve design and implantation depth. Coronary engagement after previous SAPIEN 3 (A) implantation is commonly feasible with standard catheters, and the access route may be least impaired (green arrow); engagement across the stent frame (red arrow) may become necessary only in the case of high THV positioning or low coronary take-off. Potential access routes for the ACURATE neo valve (B) may be via the stabilisation arches (red arrow) or from outside the valve frame (dotted green arrow). The common access route in tall-frame valves ([C] Portico/Navitor; [D] Evolut) is across the uncovered stent struts above the leaflet plane (red arrows), whereas, especially in patients with a wide sinotubular junction or sinus of Valsalva, an access route from outside the valve frame (dotted green arrows) may be an alternative. The lower leaflet position and the larger cells of the Portico/Navitor THV stent frame may facilitate coronary catheterisation in comparison with the Evolut platform. Regardless of the THV type, correct commissural alignment of the prosthesis will usually facilitate coronary access. THV: transcatheter heart valve](https://www.pcronline.com/var/pcrov3/storage/images/media/pcr/news/2023/journal-club-2023/management-of-coronary-artery-disease-in-patients-undergoing-transcatheter-aortic-valve-implantation/figure-4-coronary-access-according-to-valve-design-and-implantation-depth/19027984-1-eng-GB/figure-4-coronary-access-according-to-valve-design-and-implantation-depth.jpg)
Figure 4. Coronary access according to valve design and implantation depth. Coronary engagement after previous SAPIEN 3 (A) implantation is commonly feasible with standard catheters, and the access route may be least impaired (green arrow); engagement across the stent frame (red arrow) may become necessary only in the case of high THV positioning or low coronary take-off. Potential access routes for the ACURATE neo valve (B) may be via the stabilisation arches (red arrow) or from outside the valve frame (dotted green arrow). The common access route in tall-frame valves ([C] Portico/Navitor; [D] Evolut) is across the uncovered stent struts above the leaflet plane (red arrows), whereas, especially in patients with a wide sinotubular junction or sinus of Valsalva, an access route from outside the valve frame (dotted green arrows) may be an alternative. The lower leaflet position and the larger cells of the Portico/Navitor THV stent frame may facilitate coronary catheterisation in comparison with the Evolut platform. Regardless of the THV type, correct commissural alignment of the prosthesis will usually facilitate coronary access. THV: transcatheter heart valve
Source: EuroIntervention Journal
It should be noted that the THV leaflet height with respect to the recommended annular positioning, which is lowest for the SAPIEN 3/Ultra and highest for the Evolut R/Pro THVs, influences coronary access after TAVI (and after redo-TAVI) more than the commissural post height (Figure 3). Notably, coronary access after TAVI is also influenced by plenty of essential anatomical factors, including sinus sizes (particularly in relation to THV size), sinotubular junction (STJ) height and width, and coronary location in terms of height and relation with native commissures. Notwithstanding, there is growing evidence in the literature that high stent-frame THVs with a supra-annular leaflet position make selective coronary cannulation more challenging, particularly in the case of severe commissural misalignment or a high implant THV position, the latter being pursued for most THV types to reduce rates of permanent pacemaker implantation.
To avoid overlap with the coronary ostia and maintain optimal coronary access, the THV commissures should be aligned with the native aortic valve commissures. When implanting a tall-frame THV, commissural alignment should always be pursued.
To improve commissural alignment during TAVI, the following principles should be followed:
- Preprocedurally, a CT scan is used to identify a patient-specific fluoroscopic projection (C-arm angulation) in which the native aortic valve commissures can be identified - specifically, the right and left (RL) coronary cusp overlap view or the 3-cusp coplanar view (Figure 5).
- Knowing the THV-specific fluoroscopic markers that allow identification of prosthetic neo-commissures when the bioprosthesis is still crimped prior to deployment (Figure 6).
- To understand the possible THV orientation manoeuvres during deployment, so that one of the neo-commissures is placed where the right and left coronary cusps overlap (Figure 7).
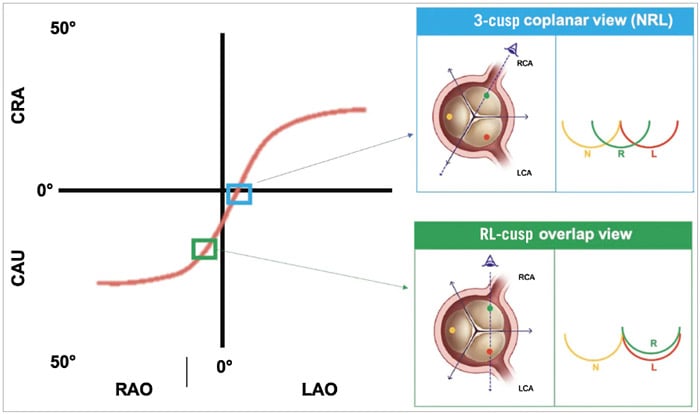
Figure 5. The S-curve of different fluoroscopic working views for transcatheter aortic valve deployment. The S-curve identifies infinite patient-specific fluoroscopic projections in which the 3 aortic cusps are aligned on the same plane. Among them, there are 3 projections where the 3 cusps are equidistant (NRL, LNR and RLN) and 3 other projections where 2 of 3 cusps are overlapping (RL cusp overlap, LN cusp overlap, NR cusp overlap). The most widely used implanting views are the 3-cusp coplanar view NRL and the RL cusp overlap view. CAU: caudal; CRA: cranial; L: left coronary cusp; LAO: left anterior oblique; LCA: left coronary artery; N: non-coronary cusp; R: right coronary cusp; RAO: right anterior oblique; RCA: right coronary artery.
Source: EuroIntervention Journal
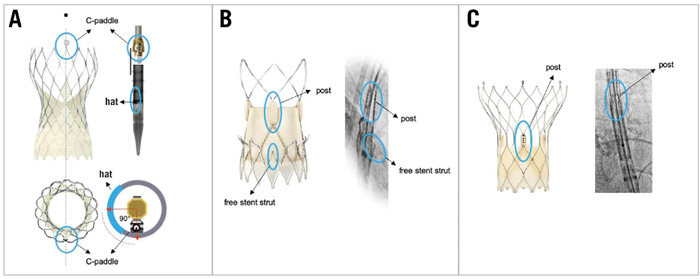
Figure 6. THV-specific fluoroscopic markers of the THV commissural posts. THV-specific fluoroscopic markers identifying prosthetic neocommissures in different types of expanded and crimped THVs. A) CoreValve Evolut platform; B) ACURATE neo2 platform; C) Portico/Navitor platform. THV: transcatheter heart valve.
Source: EuroIntervention Journal
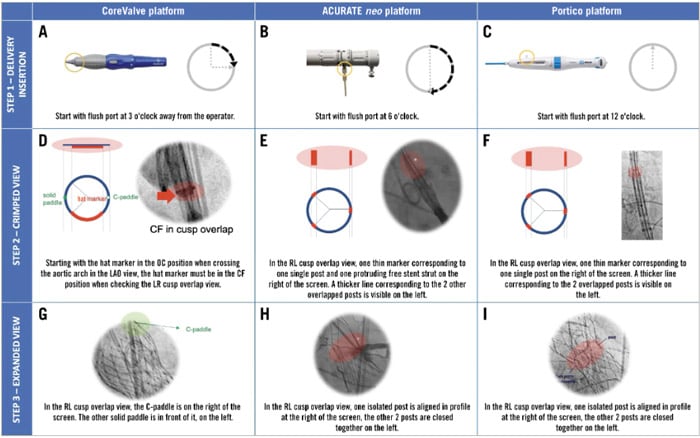
Figure 7. Implantation steps to obtain commissural alignment with different type of THVs. The figure shows the suggested rotation of the delivery system at the time of insertion (A, B, C), the expected fluoroscopic view of radiopaque markers in the RL cusp overlap view when the THV is still crimped (D, E, F) and the final fluoroscopic view after valve deployment in the same RL cusp overlap view (G, H, I) when implanting different THVs pursuing commissural alignment. If, before valve deployment, the fluoroscopic view is different from expected in the cusp overlap view, the operator should slowly torque the delivery catheter clockwise in order to obtain the desired orientation. Even if a gentle torque could be attempted with the THV at the level of the aortic valve, with the CoreValve platform, it is advisable to perform this manoeuvre only in the descending aorta to facilitate torquing force transmission through the delivery system. CF: central front; LAO: left anterior oblique; OC: outer curve; THV: transcatheter heart valve.
Source: EuroIntervention Journal
The difficulty of engaging the coronary ostia depends on aortic root and STJ anatomy, sinus sizes, coronary takeoff height and position, THV design and height, and commissural alignment. Preprocedural planning is critical when THVs are implanted in a partially supracoronary position, which is most common when the implant position is high, and/or in patients with a shallow sinus of Valsalva and a low coronary take-off height.
The strategies listed below may be considered:
- The use of the left radial or transfemoral approach may aid in traversing the stent frame.
- Smaller catheter sizes, such as Judkins Left 3.5 or 4.0, Judkins Right 4.0, Amplatz 2.0 (Cordis), Ikari 1.0 (or 1.5) (Terumo), or 3DRC (Medtronic), work best for selective engagement.
- Intracoronary guidewires enable selective intubation in cases where access is difficult. If PCI is required, catheter extensions can help with nonselective cannulation.
Coronary access after TAVI-in-TAVI
With the expansion of TAVI to lower-risk and younger patients, an increasing number of patients are likely to surpass THV durability and require redo-TAVI. Coronary access impairment following TAVI-in-TAVI will be determined by the coronary take-off height in relation to the neoskirt, and the distance between the THV stent frame and the aortic wall above the coronary ostia.
Figure 8 represents various coronary access scenarios following TAVI-in-TAVI.

Figure 8. Coronary access after TAVI-in-TAVI with different combinations of SAPIEN and CoreValve/Evolut transcatheter heart valves, depending on aortic root anatomy. STJ: sinotubular junction; TAVI: transcatheter aortic valve implantation.
Source: EuroIntervention Journal
In scenario C, where coronary ostia are located below the neoskirt at a small sinotubular junction, and the distance between the stent frame and the aortic wall is 2 mm, implantation of a second THV will make coronary access impossible. This scenario is more likely if a high stent frame with supra-annular leaflets is implanted during the first TAVI.
Conclusions
- Because CAD and AS frequently coexist, evaluating and managing CAD in TAVI candidates is critical, especially with the procedure's expansion to younger and lower-risk patients.
- Although invasive coronary angiography is still the gold standard for CAD diagnosis, CTCA may be considered for initial screening, especially in patients at low risk for CAD. The role of coronary invasive physiology evaluation needs to be clarified further.
- PCI should be performed in TAVI patients with severe CAD involving proximal vessel segments or in patients with angina, preferably before THV implantation, and especially if a THV with supra-annular leaflets is chosen.
- Regardless of the THV type, commissural alignment techniques should be used on a regular basis to improve coronary