25 May 2023
A new resorbable magnesium scaffold for de novo coronary lesions (DREAMS 3G): one-year results of the BIOMAG-1 first-in-human study
Selected in EuroIntervention Journal by P. Xaplanteris , L. Koliastasis
The 12-month outcomes of the BIOMAG-I first-in-human trial demonstrate a favorable safety profile for the third-generation magnesium scaffold and 38 % improved performance compared to its precursor.
References
Authors
Michael Haude, Adrian Wlodarczak, René J van der Schaaf, Jan Torzewski, Bert Ferdinande, Javier Escaned, Juan F Iglesias, Johan Bennett, Gabor G. Toth, Michael Joner, Ralph Toelg, Marcus Wiemer, Göran Olivecrano, Paul Vermeersch, Hector M. Garcia-Garcia, Ron Waksman
Reference
DOI: 10.4244/EIJ-D-23-00326
Published
May 17, 2023
Link
Read the abstract
Reviewers
Our Comment
Source: EuroIntervention Journal
Why this study – the rationale/objective?
The attractive idea of scaffolding the coronary vessels without permanent metallic endoprosthesis was materialized by the reabsorbable stents. They protect the endothelium during the initial healing phase, but while absorbed support the original mechanical and hydraulic properties of the vessel.
Second-generation magnesium reabsorbable stents were not as effective as contemporary drug eluting stents (DES), and the third-generation (DREAMS 3G) was developed and studied in the first-in-human clinical trial BIOMAG-I, demonstrating better outcomes comparable to DES.
DREAMS 3G scaffold was designed with reduced strut thickness down to 99 μm, targeting less surface area and new magnesium alloy, aiming in better stability and absorption homogeneity.
The 12-month safety and performance findings were announced at EuroPCR 2023 in the late breaking trials session with simultaneous publication in Eurointervention.
How was it executed? - the methodology
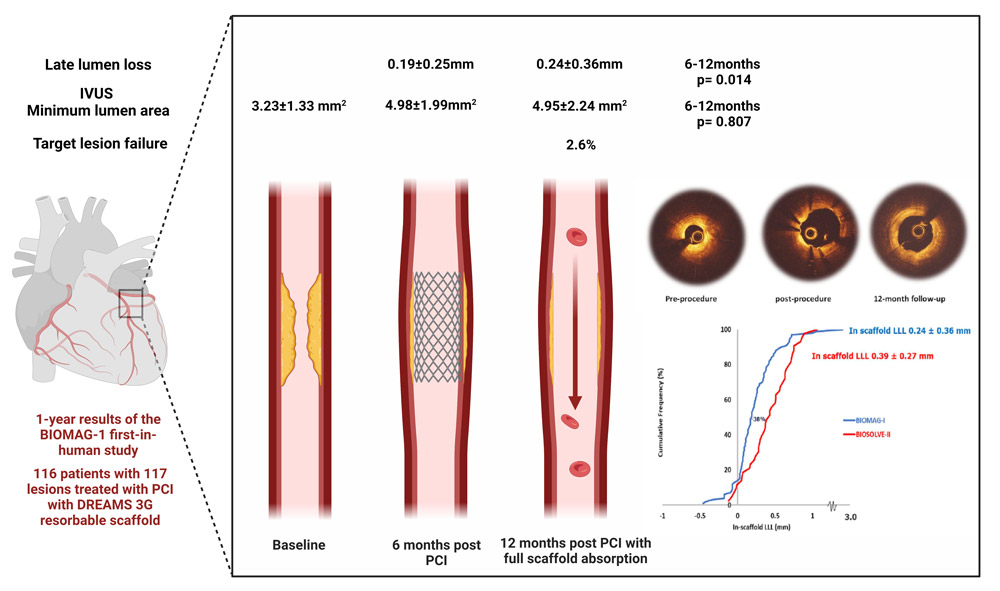
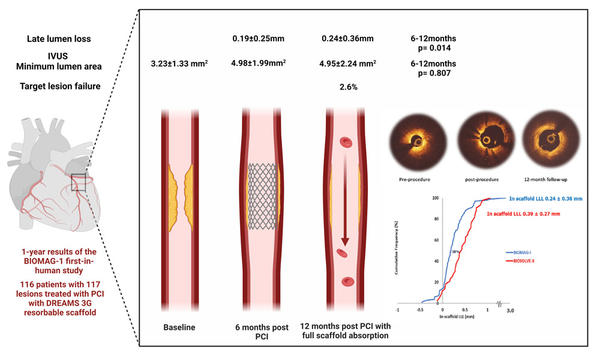
This prospective, multicenter single-arm study included 116 patients with symptomatic coronary artery disease treated with DREAMS 3G (“Magmaris”) reabsorbable scaffolds. 6-month results were promising, but complete reabsorption was designed to occur in 12 months.
A “4P-strategy” was followed with adequate patient selection, proper sizing, adequate pre- and post-dilatation in every patient following the preliminary Magmaris recommendations.
In-stent late-lumen-loss (LLL) calculated with quantitative coronary angiography (QCA) was the primary endpoint, and secondary endpoints included angiographic and intravascular imaging measurements. Clinical endpoints were target lesion failure, cardiac death, target vessel myocardial infarction, target lesion revascularization and stent thrombosis.
What is the main result?
The key points of the study are the following:
- The primary endpoint of in-scaffold LLL was 0.24 ± 0.36 mm (median 0.19, IQR: 0.06–0.36) in 100 patients’ serial data, whereas 6-month LLL was 0.19 ± 0.25 (6-12 months p = 0.014) achieving better outcomes than the BIOSOLVE-II trial and laying in the range of contemporary DES performance (DES LLL results at 9 months).
- IVUS 12-month minimum lumen area was 4.95 ± 2.24 mm2 from pre-procedural 3.23 ± 1.33 mm2 (12 months p <0.0001) and comparable to 6-month (6-12 months p = 0.807).
- Target lesion failure was documented in 3 patients (2.6 %) that underwent clinically-driven target lesion revascularization.
- No cardiac death, target-vessel myocardial infarction or index stent thrombosis was reported at 12-months, despite 6-month dual antiplatelet therapy duration.
Critical reading and the relevance for clinical practice
The 12-month outcomes of the BIOMAG-I first-in-human trial demonstrate a favorable safety profile for the third-generation magnesium scaffold, and 38 % improved performance compared to its precursor.
With the caveat of a small population sample, the adherence to the 4P-strategy and the exclusion of very complex lesions, the DREAMS 3G stent seems to meet its design goals.
A longer follow-up, as well as outcomes with the new scaffold in real world practice, and more complex anatomical scenarios, is warranted in order to confirm its safety profile and avoid a hasty introduction in the market.
Of note, the use of intracoronary imaging is a prerequisite for scaffold implantation; OCT/IVUS should be available and operators should be familiar with these modalities, something not always given in all cathlab settings.
To go further...
Watch the interview with Michael Haude, one of the co-authors of the BIOMAG-1 Trial, given by Luis Ortega Paz to find out more!