04 Nov 2024
Comparison of strategies for vascular ACCESS closure after transcatheter aortic valve implantation: the ACCESS-TAVI randomized trial
Selected in European Heart Journal by S. Brugaletta
The present study explores the alternative of 1 suture-based + 1 plug closure device vs. the conventional one, finding a superiority of this approach in achieving the full closure, hemostasis with a reduced incidence of complications.
References
Authors
Tobias Rheude, Hendrik Ruge, Niklas Altaner, Costanza Pellegrini, Hector Alvarez Covarrubias, N Patrick Mayr, Salvatore Cassese, Sebastian Kufner, Yousuke Taniguchi, Christian Thilo, Markus Klos, Magdalena Erlebach, Simon Schneider, Martin Jurisic, Karl-Ludwig Laugwitz, Rüdiger Lange, Heribert Schunkert, Adnan Kastrati, Markus Krane, Erion Xhepa, Michael Joner
Reference
European Heart Journal, ehae784, https://doi.org/10.1093/eurheartj/ehae784
Published
30 October 2024
Link
Read the abstractReviewer
My Comment
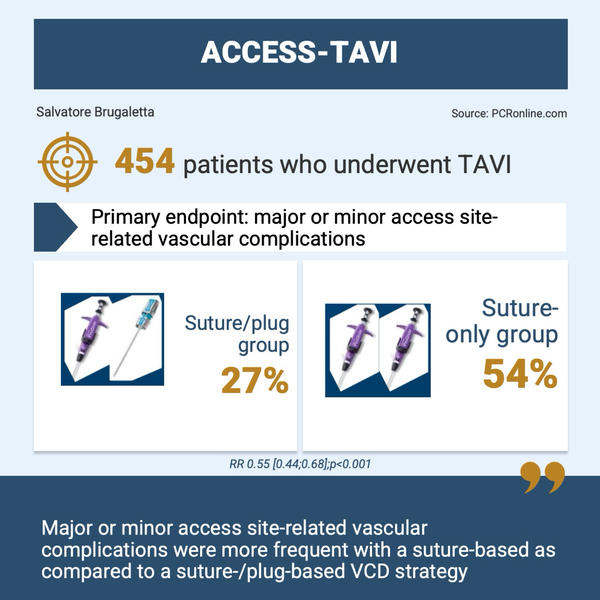
Infographic presenting the key information from the ACCESS-TAVI study.
Source: PCRonline.com

Infographic presenting the key information from the ACCESS-TAVI study.
Source: PCRonline.com
Why this study – the rationale/objective?
Data from randomized trials investigating different access closure strategies after transfemoral transcatheter aortic valve implantation (TF-TAVI) remain scarce.
In this study, two vascular closure device (VCD) strategies to achieve hemostasis after TF-TAVI were compared.
How was it executed – the methodology?
The ACCESS-TAVI (Comparison of Strategies for Vascular ACCESS Closure after Transcatheter Aortic Valve Implantation) is a prospective, multicenter trial in which patients undergoing TF-TAVI were randomly assigned to a strategy with a
combined suture-/plug-based VCD strategy (suture/plug group) using one ProGlide™/ProStyle™ (Abbott Vascular) and one Angio-Seal® (Terumo) versus a suture-based VCD strategy (suture-only group) using two ProGlides™/ProStyles™.
The primary endpoint was a composite of major or minor access site-related vascular complications during index hospitalization according to Valve Academic Research Consortium (VARC)-3 criteria.
Key secondary endpoints included time to hemostasis, VARC-3 bleeding type ≥ 2 and all-cause mortality over 30 days.
What is the main result?
Between September 2022 and April 2024, 454 patients were randomized.
The primary endpoint occurred in 27 % (62/230) in the suture/plug group and 54 % (121/224) in the suture-only group (relative risk [RR] 0.55 [95 % confidence interval: 0.44;0.68]; p < 0.001).
Time to hemostasis was significantly shorter in the suture/plug group compared to the suture-only group (108 ± 208 s vs. 206 ± 171 s; p < 0.001).
At 30 days, bleeding type ≥ 2 occurred less often in the suture/plug group compared to the suture only group (6.2 % vs. 12.1 %, RR 0.66 [0.43;1.02]; p = 0.032), with no significant difference in mortality.
Critical reading and the relevance for clinical practice
Vascular access may represent the most challenging part of a TAVI procedure, especially taking into account the TAVI patients are usually old and with many comorbidities, which make femoral access difficult.
One part of this access is to ensure a complete hemostasis without complications. The most used technique for vascular closure is represented by the implantation of 2 suture-based closure devices. The present study explores the alternative of 1 suture-based + 1 plug closure device vs. the conventional one, finding a superiority of this approach in achieving the full closure, hemostasis with a reduced incidence of complications.
There are some important points to highlight:
- First it is important to see that there was a high use of echo-guided puncture, which unfortunately does not reach 100 %. Echo-guided puncture should be an important step of TAVI.
- Another important point to analyze is that in suture-based group, 40 % of the patients need an additional vascular closure device, which was in almost 60 % of the cases a plug-based device. This may mean that use of plug-based device on the top of 2 suture-based device as rescue is not so helpful as it is by intention on the top of only 1 suture-based device.
- Last, but not least use of 6F or 8F plug-based device in the VCD group was left to operator discretion, but the data on their use are not reported.
Which is the closure technique you use in your clinical practice for TAVI?






1 comment
eco-guided puncture and only one proglide