Effects of proton pump inhibitors on gastrointestinal bleeding and cardiovascular outcomes in myocardial infarction patients treated with DAPT
Selected in EuroIntervention Journal by R. Sava , N. Ryan
This large retrospective analysis using data from the Republic of Korea’s Nationwide Health Claims database showed that co-prescription of a PPI in patients treated with DAPT following an AMI was associated with reduced major GI bleeding as well as reduced minor GI bleeding requiring hospitalisation, regardless of the type of P2Y12 inhibitor prescribed.
References
Authors
Kang Danbee,Choi Ki,Park Hyejeong,Heo Jihye,Park Taek,Lee Joo,Cho Juhee,Yang Jeong,Song Young,Choi Seung-Hyuk,Gwon Hyeon-Cheol,Hahn Joo-Yong
Reference
10.4244/EIJ-D-24-00673 • Feb 17, 2025
Published
Feb 17, 2025
Link
Read the abstractReviewers
Our Comment
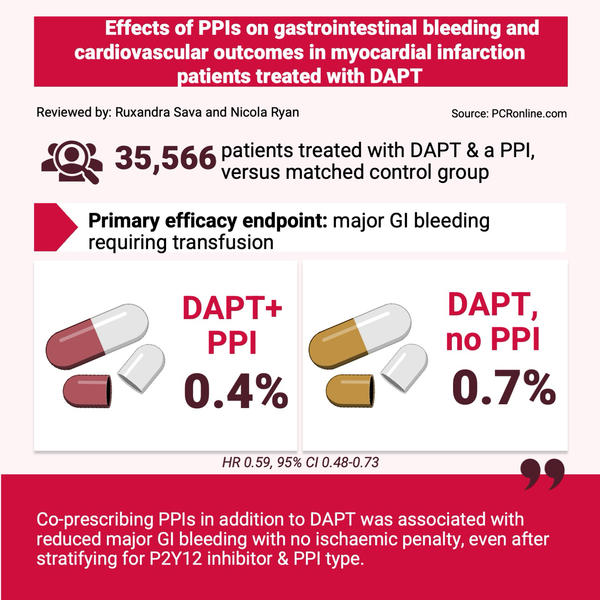
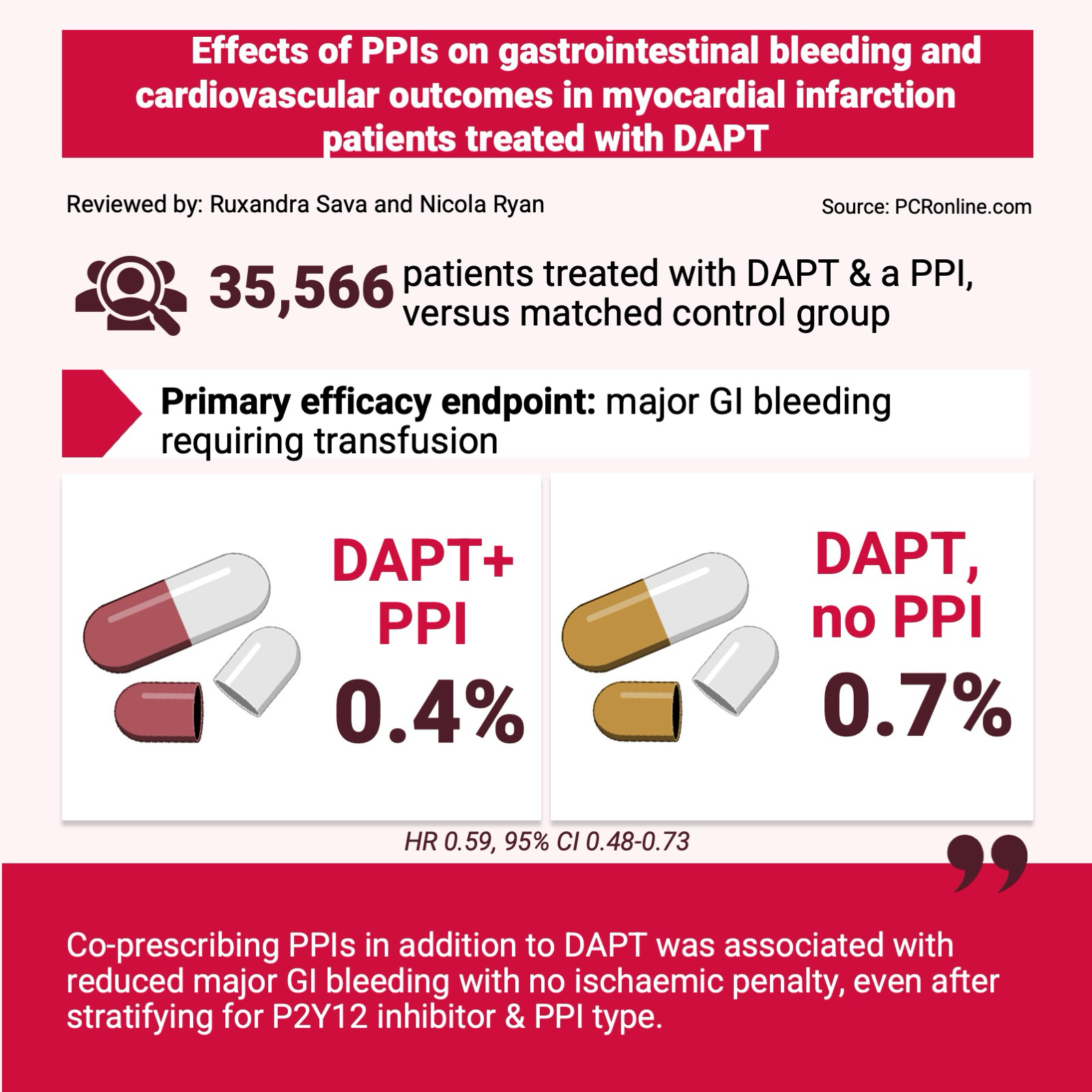
Reviewed by: Ruxandra Sava and Nicola Ryan
Source: PCRonline.com
Why this study – the rationale/objective?
In patients treated with dual antiplatelet therapy (DAPT), concern persists regarding the potential of proton pump inhibitors (PPIs) to induce ischemic events. There is also conflicting evidence regarding the effectiveness of PPIs to decrease gastrointestinal bleeding.
The present study investigated whether the co-prescription of a PPI and DAPT in patients with acute myocardial infarction (AMI) treated with percutaneous coronary intervention (PCI) was associated with gastrointestinal bleeding or ischemic events.
How was it executed - the methodology
This study was a retrospective analysis of the National Health Claims of Korea. From 52 million Korean citizens, the investigators selected patients aged 40-80 years old who underwent PCI for AMI and were treated with a combination of aspirin and either Clopidogrel, Prasugrel or Ticagrelor.
Outcomes were compared between patients treated by DAPT with and without PPI co-treatment. Propensity score matching was used to minimise differences between baseline characteristics among groups. The analysis was based on intention-to-treat – i.e., based on discharge prescriptions. Patients were followed up until outcome occurrence or up to one year.
The study aimed to emulate a randomised trial. Exclusion criteria were similar to the COGENT-1 trial, the most significant being: patients prescribed a PPI, an H2-receptor antagonist, Sucralfate or Misoprostol within 30 days of admission, co-prescriptions of oral anticoagulants and patients with a history of red blood cell transfusions.
- The primary efficacy endpoint was major GI bleeding requiring transfusion.
- The secondary efficacy endpoint included major or minor GI bleeding requiring hospitalisation, regardless of transfusion status.
- The primary safety endpoint was major adverse cardiac and cerebrovascular events (MACCE), defined as a composite of cardiovascular death, spontaneous MI, repeat revascularisation, and ischaemic stroke.
What is the main result?
35,566 patients were prescribed a PPI alongside DAPT. The average age was 61.2 years, male sex was predominant (83.2 %) and 23.3 % of patients were at high bleeding risk based on the ARC-HBR score. These patients were matched using propensity scoring with patients on DAPT without PPI.
Patients treated by DAPT with Prasugrel or Ticagrelor were more frequently co-prescribed a PPI. Specifically, 44.8 % of patients in the PPI group were treated with Ticagrelor (vs. 33.1 % in the group without PPI), and 8.8 % were treated with Prasugrel (vs 6.6 %). The opposite was true for patient treated with Clopidogrel, who were more frequently in the non-PPI group (60.3 % vs 47.2 %).
Compliance, assessed as patients who were alive and continued to be prescribed a PPIs, aspirin or a P2Y12 inhibitors at 1 year, was 55.2 %, 52.6 %, and 50.0 %, respectively.
- The primary efficacy endpoint demonstrated that PPI co-prescription was associated with a significantly lower rate of major GI bleeding (0.7 % vs 0.4 %, HR 0.59, 95 % CI 0.48-0.73).
- PPI co-treatment was associated with a reduced rate of major GI bleeding irrespective of DAPT with clopidogrel (0.4. vs 0.6 %, HR 0.62, 95 % CI 0.45-0.86) or with Prasugrel/Ticagrelor (0.5 vs 0.8 %, HR 0.58, 95 % CI 0.44-0.76).
- PPI treatment was also associated with a lower rate of the secondary efficacy endpoint (HR 0.7, 95 % CI 0.6-0.83).
- There was no difference between groups in the primary safety endpoint (13.1 vs 13.3 %, HR 0.98, 95 % CI 0.94-1.02).
Critical reading and the relevance for clinical practice
This large retrospective analysis using data from the Republic of Korea’s Nationwide Health Claims database showed that co-prescription of a PPI in patients treated with DAPT following an AMI was associated with reduced major GI bleeding as well as reduced minor GI bleeding requiring hospitalisation, regardless of the type of P2Y12 inhibitor prescribed.
Subgroup analysis confirmed the benefit of PPI treatment regardless of type of P2Y12 inhibitor, high-bleeding risk or history of GI ulcer. Moreover, this appears to be a class effect, as different PPIs were associated with a similar protective effect. The protective effect was maintained in patients at high bleeding risk, i.e. those at high ARB-HBR scores and those with a previous diagnosis of GI ulcer. This effect was amplified by increased compliance with PPI treatment, as demonstrated by a per-protocol analysis including only patients highly compliant with PPI treatment versus no PPI use (HR 0.20, 95 % CI 0.16-0.27).
The study adds reassurance regarding the safety of PPI and DAPT co-prescriptions, as it did not highlight any signal of increased ischemic events. To investigate the interaction between the safety outcome, PPI and individual PY12 inhibitors, a stratified analysis was conducted. In patients treated with Clopidogrel, PPI co-treatment did not negatively impact the safety endpoint (14.2 % vs 13.8 %, HR 0.98, 95 % CI 0.92-1.04), and there was no difference in rates of spontaneous myocardial infarction (6.7 vs 6.7 %, HR 1.01, HR 0.93-1.1), repeat revascularisation (7.9 % vs 8.1 %, HR 0.97, 95 % CI 0.9-1.05) or cardiovascular death (1.1 vs 1.1 %, HR 0.97, 95 % CI 0.79-1.19). Similarly, no differences were observed in patients prescribed Prasugrel or Ticagrelor.
Strengths of the study include the large real-world dataset, inclusion of a control group matched by propensity score, and the choice of clinically relevant endpoints. However, there are important limitations. In addition to its retrospective nature and reliance on medical diagnostic coding, compliance with PPI was estimated based on medical prescriptions. Whilst the importance of adhering to the DAPT treatment is likely emphasised by prescribing physicians due to the risk of stent thrombosis, lack of adherence to PPI may be more frequent, and could potentially obscure a real increase in ischemic cardiovascular events. Moreover, it is unclear why compliance with DAPT at 1 year was only approximately 50 % for aspirin or P2Y12 inhibitors, which is surprising, especially as only 20 % of patients were estimated to be at high bleeding risk using the ARC-HBR criteria.
The findings complement those of the COGENT trial1, a randomised control trial of Omeprazole vs placebo administered to patients undergoing DAPT with aspirin and Clopidogrel. With a sample size of 3,873 patients, the trial demonstrated reduced GI bleeding events and no increase in ischemic events in patients treated with Omeprazole. However, the trial was limited by premature termination due to budget constraints and a follow-up of only 6 months. While ischemic events arising after 6 months may have been missed in COGENT, the ischemic risk is highest in the first months after stent implantation, before stent endothelialisation is completed2. The pharmacologic mechanism underpinning clopidogrel – PPI interaction is inhibition of CYP2C19, the enzyme responsible for activating Clopidogrel, mainly by drugs such as Omeprazole and Esomeprazole3,4. Prescription of an alternative PPIs may be considered in patients treated with Clopidogrel.
ESC and ACC/AHA guideline recommendations for PPI co-treatment in patients receiving DAPT have recently been harmonised. While the 2017 ESC focused update on dual antiplatelet therapy in coronary artery disease recommended PPI treatment in all patients treated with DAPT3, the more recent 2023 ESC Guidelines5 and the 2025 ACC/AHA/ACEP/NAEMSP/SCAI Guidelines for the Management of Patients With Acute Coronary Syndromes6 both recommend PPI treatment only in patients at high risk of GI bleeding (class I, level of evidence A). However, the present study found no evidence of interaction between high bleeding risk and the efficacy endpoint, suggesting that some patients at risk for GI bleeding may be missed by current risk scores. Current evidence suggests that, in patients treated with DAPT that are considered at high or higher bleeding risk, the threshold for co-prescription of a PPI inhibitor should be low. Risk scores such as ARC-HBR can aid in evaluation of a high bleeding risk, but clinical judgement remains paramount.
References
- Bhatt DL, Cryer BL, Contant CF, et al. Clopidogrel with or without Omeprazole in Coronary Artery Disease. N Engl J Med. 2010;363(20):1909-1917. doi:10.1056/NEJMoa1007964
- Chau KH, Kirtane AJ, Easterwood RM, et al. Stent Thrombosis Risk Over Time on the Basis of Clinical Presentation and Platelet Reactivity: Analysis From ADAPT-DES. JACC Cardiovasc Interv. 2021;14(4):417-427. doi:10.1016/j.jcin.2020.12.005
- Valgimigli M, Bueno H, Byrne RA, et al. 2017 ESC focused update on dual antiplatelet therapy in coronary artery disease developed in collaboration with EACTS: The Task Force for dual antiplatelet therapy in coronary artery disease of the European Society of Cardiology (ESC) and of the European Association for Cardio-Thoracic Surgery (EACTS). Eur Heart J. 2018;39(3):213-260. doi:10.1093/eurheartj/ehx419
- Shah NH, LePendu P, Bauer-Mehren A, et al. Proton Pump Inhibitor Usage and the Risk of Myocardial Infarction in the General Population. PLOS ONE. 2015;10(6):e0124653. doi:10.1371/journal.pone.0124653
- Byrne RA, Rossello X, Coughlan JJ, et al. 2023 ESC Guidelines for the management of acute coronary syndromes: Developed by the task force on the management of acute coronary syndromes of the European Society of Cardiology (ESC). Eur Heart J. 2023;44(38):3720-3826. doi:10.1093/eurheartj/ehad191
- Rao SV, O ’Donoghue Michelle L., Ruel M, et al. 2025 ACC/AHA/ACEP/NAEMSP/SCAI Guideline for the Management of Patients With Acute Coronary Syndromes. JACC. 0(0). doi:10.1016/j.jacc.2024.11.009





No comments yet!