Impact of transcatheter or surgical aortic valve performance on 5-year outcomes in patients at ≥ intermediate risk
Selected in JACC by A. Sticchi
The objective of this study was to validate standardised clinical criteria for BVD and evaluate its association with mortality and rehospitalisation, thereby guiding optimal valve selection in clinical practice.
References
Authors
Steven J. Yakubov, Nicolas M. Van Mieghem, Jae K. Oh, Saki Ito, Kendra J. Grubb, Daniel O’Hair, John K. Forrest, Hemal Gada, Mubashir Mumtaz, G. Michael Deeb, Gilbert H.L. Tang, Joshua D. Rovin, Renuka Jain, Stephan Windecker, Kimberly A. Skelding, Neal S. Kleiman, Stanley J. Chetcuti, Alexandra Dedrick, Sarah Verdoliva Boatman, Jeffrey J. Popma, and Michael J. Reardon
Reference
JACC. Mar 09, 2025. Epublished DOI: 10.1016/j.jacc.2025.02.009
Published
Mar 09, 2025
Link
Read the abstractReviewer
My Comment
Designed by Alessandro Sticchi. Source: PCRonline
Why this study – the rationale/objective?
This study was undertaken to address a critical gap in understanding the long‐term durability and clinical impact of bioprosthetic valve dysfunction (BVD) in patients with severe aortic stenosis.
With lifetime management becoming a key consideration—especially as transcatheter aortic valve replacement (TAVR) extends to lower-risk and younger patients—the study aimed to compare 5-year outcomes between CoreValve/Evolut TAVR and surgical aortic valve replacement (SAVR).
The objective was to validate standardised clinical criteria for BVD and evaluate its association with mortality and rehospitalisation, thereby guiding optimal valve selection in clinical practice.
How was it executed – the methodology?
The study was executed as a rigorous post-hoc pooled analysis, combining data from multiple large-scale investigations.
Key execution details include:
- Data Sources:
- Two major randomised controlled trials (RCTs): the US High Risk Pivotal trial and the SURTAVI trial, involving 2,344 patients (1,227 undergoing CoreValve/Evolut TAVI and 1,117 receiving SAVR).
- Additional data from the Extreme Risk Pivotal trial (608 TAVI patients) and the CoreValve Continued Access Study (2,654 TAVI patients).
- Population and Assessments:
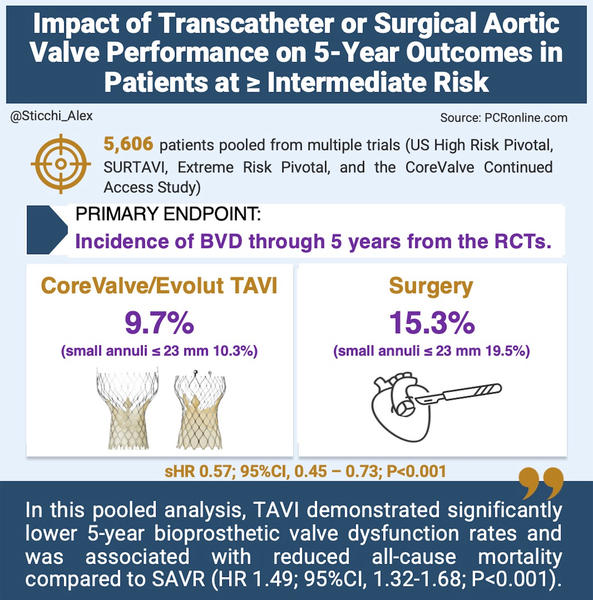
- A total of 5,606 patients were evaluable for bioprosthetic valve dysfunction (BVD).
- Standardised definitions based on VARC-3 criteria were applied to classify BVD components, including structural valve deterioration (SVD), non-structural valve dysfunction (NSVD) (with subcomponents such as severe prosthesis–patient mismatch [PPM] and paravalvular regurgitation [PVR]), clinical valve thrombosis, and infectious endocarditis.
- Echocardiographic assessments were performed at baseline, discharge, 30 days, 6 months, 12 months, and then annually through 5 years.
- Statistical Analysis:
- Advanced statistical techniques were employed, such as Fine-Gray regression to account for competing risks and multivariable Cox proportional hazards models.
- Adjustments were made for important covariates including age, STS-PROM, New York Heart Association (NYHA) class, and other clinical variables to ensure robust comparisons between the TAVI and SAVR cohorts.
What is the main result?
The study demonstrated a clear advantage for CoreValve/Evolut TAVI over SAVR in terms of mid-term valve performance, with detailed results as follows:
- Overall BVD rates:
- 5-year BVD incidence was 9.7 % in the TAVI group versus 15.3 % in the SAVR group.
- This corresponds to a sub-distribution hazard ratio (sHR) of 0.57 (95 % CI, 0.45–0.73; P < 0.001).
- Subgroup analysis by aortic annulus size:
- For patients with smaller aortic annuli (CT-derived annular diameter ≤ 23 mm):
- TAVI: 10.3 % BVD rate
- SAVR: 19.5 % BVD rate
- sHR of 0.41 (95 % CI, 0.25–0.68; P < 0.001)
- For patients with larger annuli:
- TAVI: 9.8 % BVD rate
- SAVR: 14.0 % BVD rate
- sHR of 0.65 (95 % CI, 0.49–0.86; P = 0.003)
- For patients with smaller aortic annuli (CT-derived annular diameter ≤ 23 mm):
- Component-Specific Findings:
- Structural Valve Deterioration (SVD):
- Occurred in 2.4 % of TAVI patients versus 4.5 % of SAVR patients (sHR, 0.54; P = 0.01).
- Non-Structural Valve Dysfunction (NSVD):
- Occurred in 5.4 % of TAVI patients versus 9.5 % of SAVR patients (sHR, 0.55; P < 0.001).
- Severe Prosthesis–Patient Mismatch (PPM) at 30 Days:
- Observed in 3.7 % of TAVI patients compared to 12.1 % in the SAVR group (OR, 0.28; P < 0.001).
- Structural Valve Deterioration (SVD):
- Other Findings:
- Rates of clinical valve thrombosis and infectious endocarditis were comparable between the two treatment modalities.
These precise numbers confirm that the TAVI approach not only reduces the overall risk of BVD, but also significantly lowers the risk for its individual components, ultimately translating into better mid-term clinical outcomes.
Critical reading and the relevance for clinical practice
The study offers several critical insights that can be directly applied in clinical decision-making. It robustly validates standardised VARC-3 (and by extension, EAPCI) criteria for assessing bioprosthetic valve dysfunction (BVD), establishing these definitions as reliable benchmarks for predicting mid-term adverse outcomes and supporting a uniform approach to evaluating valve performance in both comparative studies and routine clinical practice.
The data also indicate that BVD is independently associated with a 49 % increase in all-cause mortality, with even higher hazards for cardiovascular mortality and rehospitalisation for valve disease or heart failure—an effect especially pronounced in patients with smaller aortic annuli, where precise valve sizing and optimised hemodynamics are paramount.
Moreover, the findings reveal that CoreValve/Evolut TAVI exhibits superior mid-term durability compared to SAVR, as evidenced by a lower overall 5-year BVD incidence (9.7 % vs. 15.3 %) and a striking difference in patients with small annuli (10.3 % vs. 19.5 %). These results inform a more personalised, strategic approach to device selection, one that considers not only immediate procedural risk but also anticipated valve longevity and the feasibility of future interventions such as TAVR-in-TAVR or SAVR-in-TAVR.
Although TAVI may impose certain limitations for reinterventions due to design considerations, its demonstrated durability makes it an attractive option for patients at high lifetime risk, especially when balancing procedural success with long-term outcomes.
It is important to note that the analysis predominantly involves intermediate- to high-risk patients and early-generation CoreValve/Evolut devices, which may not fully capture the performance of newer-generation valves. BVD rates were also not adjusted for individual follow-up duration, making comparisons with studies that report rates per 100 patient-years more challenging. These limitations underscore the need for ongoing research with extended follow-up and in broader patient populations to refine these recommendations further.
Nevertheless, incorporating these findings into clinical practice enhances patient counseling and shared decision-making, enabling clinicians to weigh both immediate procedural risks and the long-term implications of valve choice on survival and quality of life.
By adopting a comprehensive, individualised approach that integrates valve durability into lifetime management strategies, clinicians can optimise device selection and tailor management strategies based on evolving evidence—ultimately improving patient outcomes in the management of aortic stenosis.
Future studies with extended follow-up and broader patient populations are warranted to further refine these recommendations, and these insights will continue to guide clinical decision-making and ultimately enhance patient care.






No comments yet!