17 Feb 2026
Ticagrelor vs prasugrel in patients with diabetes and multivessel coronary artery disease: the TUXEDO-2 randomised clinical trial
Selected in JAMA Cardiology by R. Piccolo
The TUXEDO-2 trial sought to directly compare prasugrel versus ticagrelor in diabetic patients undergoing percutaneous coronary intervention (PCI), with the aim of clarifying whether the non-inferiority of ticagrelor could be established.
References
Authors
Sripal Bangalore, Santosh Kumar Sinha, Rakendra Singh, Ashok Kumar Parida, Rohit Mody, Rajpal Abhaichand, Darshan Banker, Aziz Khan, Arun Kalyansundaram, Nagaraja Moorthy, Kunal Mahajan, Bishav Mohan, Bhaveesh Meel, Rajpal Singh, Sanjay Porwal, Ajit Bhagwat, Charantharayail Gopalan Bahuleyan, Deepak Davidson, Sudheer Koganti, Sunil Kumar Garsa, Prafulla Kerkar, Gopala Krishna Koduru, Madhu Sreedharan, Prashant Jagtap, G. Manohar, Santosh Kumar, Priyadarshini Arambam, Nagma Khan, Varsha Koul, Krishnankutty Sudhir, Upendra Kaul, for the TUXEDO-2 India Investigators
Reference
doi: 10.1001/jamacardio.2025.5057
Published
Published online February 11, 2026
Link
Read the abstractReviewer
My Comment
Why this study – the rationale/objective?
Diabetic patients undergoing percutaneous coronary intervention (PCI) represent a particularly high-risk population, with an increased burden of multivessel disease and a heightened risk of recurrent ischemic events.
Although both prasugrel and ticagrelor are recommended P2Y12 inhibitors in acute coronary syndromes (ACS), direct comparative data in diabetic patients with multivessel coronary artery disease are limited.
The TUXEDO-2 trial sought to directly compare prasugrel versus ticagrelor in this complex subgroup undergoing PCI, with the aim of clarifying whether the non-inferiority of ticagrelor could be established.
How was it executed? The methodology
TUXEDO-2 was a multicenter, multifactorial, 2-by-2 randomised trial. Patients with diabetes mellitus and multivessel coronary artery disease undergoing PCI were randomly assigned to one of two drug-eluting stents (biodegradable-polymer sirolimus-eluting stent [Supraflex Cruz] vs. durable-polymer everolimus-eluting stent [Xience family]) and independently randomised to one of two oral P2Y12 inhibitors (prasugrel vs. ticagrelor).
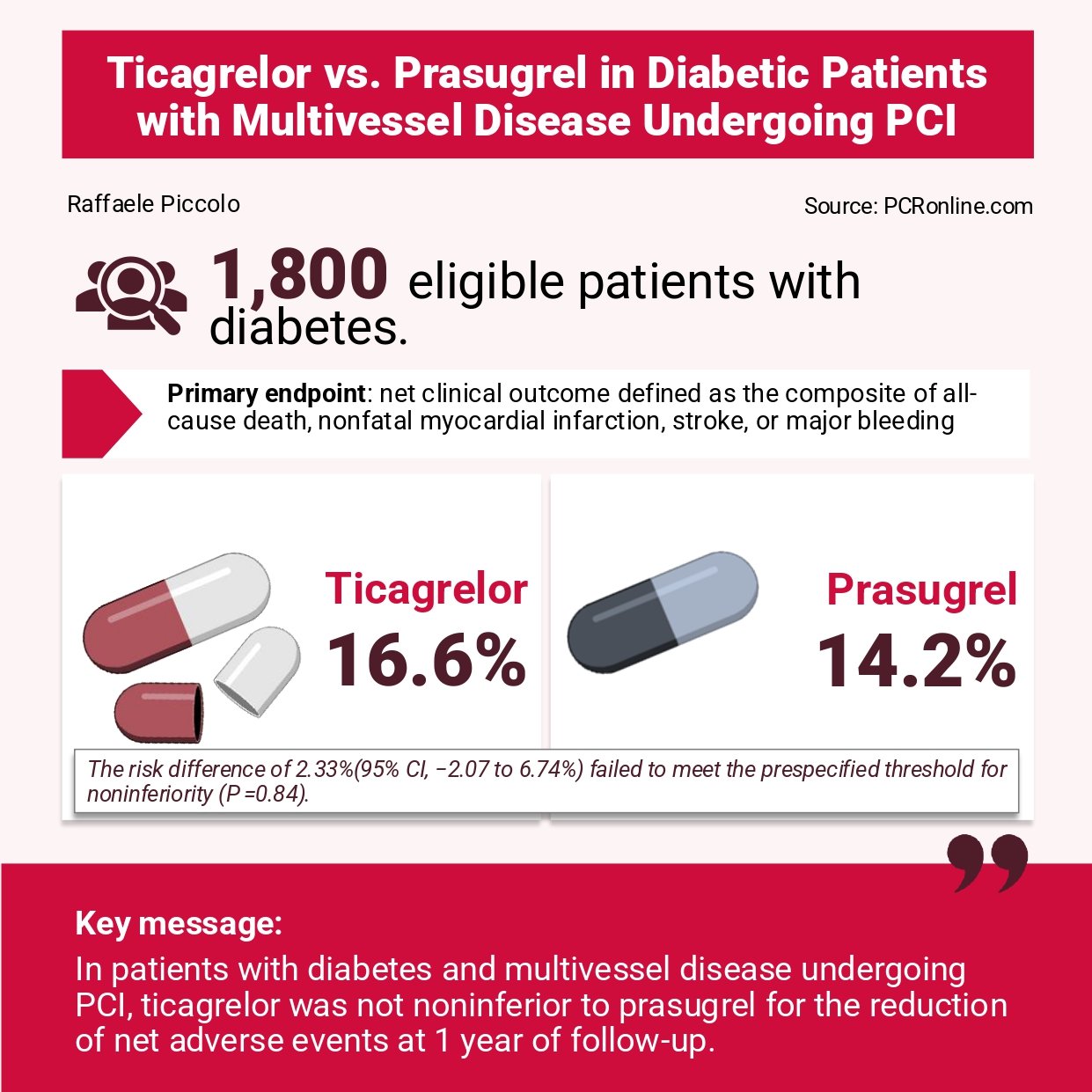
- The primary endpoint was a 1-year net clinical outcome defined as the composite of all-cause death, nonfatal myocardial infarction, stroke, or major bleeding.
- The trial was powered for a non-inferiority comparison of ticagrelor versus prasugrel, assuming an expected 15 % event rate for the primary endpoint, a 5 % non-inferiority margin, 80 % power, a one-sided alpha of 2.5 %, and an anticipated 10 % attrition rate.
What is the main result?
A total of 1,800 patients were enrolled (mean age 60 years; 28 % women). ACS was the indication for PCI in 80 % of cases. Approximately 70 % of patients underwent PCI in three vessels, and 30 % in two vessels. Radial access was used in about half of procedures.
- At 1 year, the primary endpoint occurred in 16.6 % of patients assigned to ticagrelor and 14.2 % of those assigned to prasugrel (absolute risk difference 2.33 %; 95 % confidence interval [CI] –2.07 % to 6.74 %). As the upper bound of the 95 % CI exceeded the pre-specified non-inferiority margin by +1.74 % (i.e., 6.74 % minus 5.0 %), non-inferiority of ticagrelor was not established (P = 0.12).
- Both ischemic outcomes (death, myocardial infarction, or stroke: 10.4 % vs. 8.6 %, P = 0.30) and major bleeding (BARC 3–5: 8.4 % vs. 7.1 %, P = 0.19) numerically favored prasugrel.
- Treatment effects were consistent across 13 pre-specified subgroups, without significant interaction signals.
Critical reading and the relevance for clinical practice
TUXEDO-2 addresses a clinically important and unresolved question: are prasugrel and ticagrelor truly interchangeable in high-risk PCI populations? This represents the third randomised comparison between the two agents and the first in diabetic patients.
PRAGUE-18, an earlier trial enrolling 1,230 patients with ACS, was terminated prematurely for futility and showed no difference between agents at 30 days or 1 year. In contrast, the larger ISAR-REACT 5 trial (n = 4,018) demonstrated superiority of prasugrel over ticagrelor in reducing ischemic events, with no significant difference in major bleeding. However, ISAR-REACT 5 generated debate, particularly regarding differences in drug administration timing and the imbalance in exclusions after randomisation. European guidelines subsequently provided a Class IIa recommendation favoring prasugrel over ticagrelor in ACS patients undergoing PCI, largely driven by ISAR-REACT 5. In contrast, recent American guidelines have not expressed a similar preference, reflecting ongoing uncertainty.
How should TUXEDO-2 be interpreted in this context?
Although smaller than ISAR-REACT 5, its findings align directionally with a possible advantage of prasugrel. Nevertheless, several considerations merit attention. First, pharmacodynamic studies generally show comparable levels of platelet inhibition between prasugrel and ticagrelor, making a clear biological explanation for superiority difficult to establish.
Second, in the landmark megatrials against clopidogrel (TRITON-TIMI 38 and PLATO), only ticagrelor showed a significant mortality reduction. However, PLATO has been subject to methodological critiques, including concerns regarding event adjudication patterns and accuracy of death records.
Third, in TUXEDO-2, both ischemic and bleeding events numerically favored prasugrel. This parallel direction of effect is somewhat unexpected, as a greater ischemic protection is normally offset by increased bleeding risk. The absence of a trade-off raises questions about whether the findings reflect a true pharmacologic difference, play of chance, or study-specific factors.
Taken together, TUXEDO-2 adds to the accumulating but still inconclusive body of evidence suggesting that prasugrel and ticagrelor may not be clinically equivalent in all PCI populations, particularly among high-risk diabetic patients with multivessel disease. However, the study was not powered for superiority, and confidence intervals remain wide.
Nearly two decades after TRITON-TIMI 38 and PLATO, the question of whether prasugrel is truly superior to ticagrelor remains unsettled. A large, adequately powered, contemporary randomised trial specifically designed to test superiority in well-defined high-risk subgroups would be required to provide a definitive answer.