25 Mar 2021
Coronary atherectomy via guide extension catheter for bending calcified lesions
It is challenging to treat bending calcified coronary lesions with CA. Using a guide extension catheter-supported back-up force to advance a bulky atherectomy device and a guidewire bias to the calcified lesion enabled imaging observation and sufficient dilation using a scoring balloon, which led to stent-less treatment with a DCB in some cases.
The limitations in terms of available burr size, catheter system, and procedure are reviewed and summarised in this article.
Authors
Introduction
It is challenging to treat bending calcified coronary lesions with percutaneous coronary intervention (PCI) due to difficulties in assessing lesion severity and the deployment of devices that require a strong back-up of a guiding catheter. As intravascular imaging (intravascular ultrasound (IVUS), optical coherence tomography (OCT), and optical frequency domain imaging (OFDI)) is useful in assessing the calcified thickness, arc, and longitudinal length1-3, rotational atherectomy (RA), or orbital atherectomy (OA) for calcified lesions under imaging guidance is effective in obtaining adequate stent expansion safely, which leads to fewer clinical events1,2. However, the bending calcified lesion makes it challenging to advance the imaging device and/or atherectomy catheter distally and increases the risk of coronary perforation during atherectomy.
A guide extension catheter (GEC) is useful for deploying devices in severely tight lesions with tortuosity. A few cases of RA or OA via the GEC have been reported4-6; however, its feasibility and methodology have not yet been fully established. In addition, there is only one report on the completion of atherectomy under imaging guidance via the GEC4. In this article, we sought to clarify the feasibility and efficacy of this technique under complete imaging guidance and propose an optimal method.
Methods
Five cases of bending calcified lesions were attempted to be treated with coronary atherectomy via GEC due to failure in deploying an atherectomy catheter without support (Supplementary Movie 1) at the Kyushu Medical Centre from December 2017 to October 2020.
All lesions presented obvious calcification on fluoroscopy, one or more bending lesions with an acute angle of > 90°, and difficulty in deploying an atherectomy catheter without the GEC. All procedures were successfully performed using a 7Fr guiding system and imaging observation. OCT (Dragonfly, Ilumien, Abbott Vascular, Santa Clare, CA, USA), OFDI (FastView, Lunawave, Terumo, Tokyo, Japan), or IVUS (Opticross, Polaris, Boston Scientific, Natick, MA, USA), before and after PCI.
In cases where an imaging catheter could not be advanced through the calcified lesion, the imaging observation after the first pass of the atherectomy catheter was regarded as the baseline assessment. Treatment with a drug-eluting stent (DES) or drug-coated balloon (DCB) was performed at the operator’s discretion. All patients received dual antiplatelet therapy with 81-200 mg aspirin daily and 75 mg clopidogrel or 3.75 mg prasugrel daily, which was continued for ≥ 6 months.
Imaging analysis
Imaging analysis was performed independently using the default software in each imaging machine. The lumen and external elastic membrane contours were imaged at the proximal and distal references and minimal lumen area (MLA) sites pre-PCI and post-PCI. The expansion index was defined as the percentage of lumen cross-sectional area (CSA) at the post-PCI MLA site to the average lumen CSA at the proximal and distal references7.
Results
Patient background, PCI procedure, and imaging analysis of all cases are listed in Table 1, and coronary angiography/intravascular imaging are displayed in Figure 1.
Table 1. Case presentation of coronary atherectomy via guide-extension catheter and imaging analysis
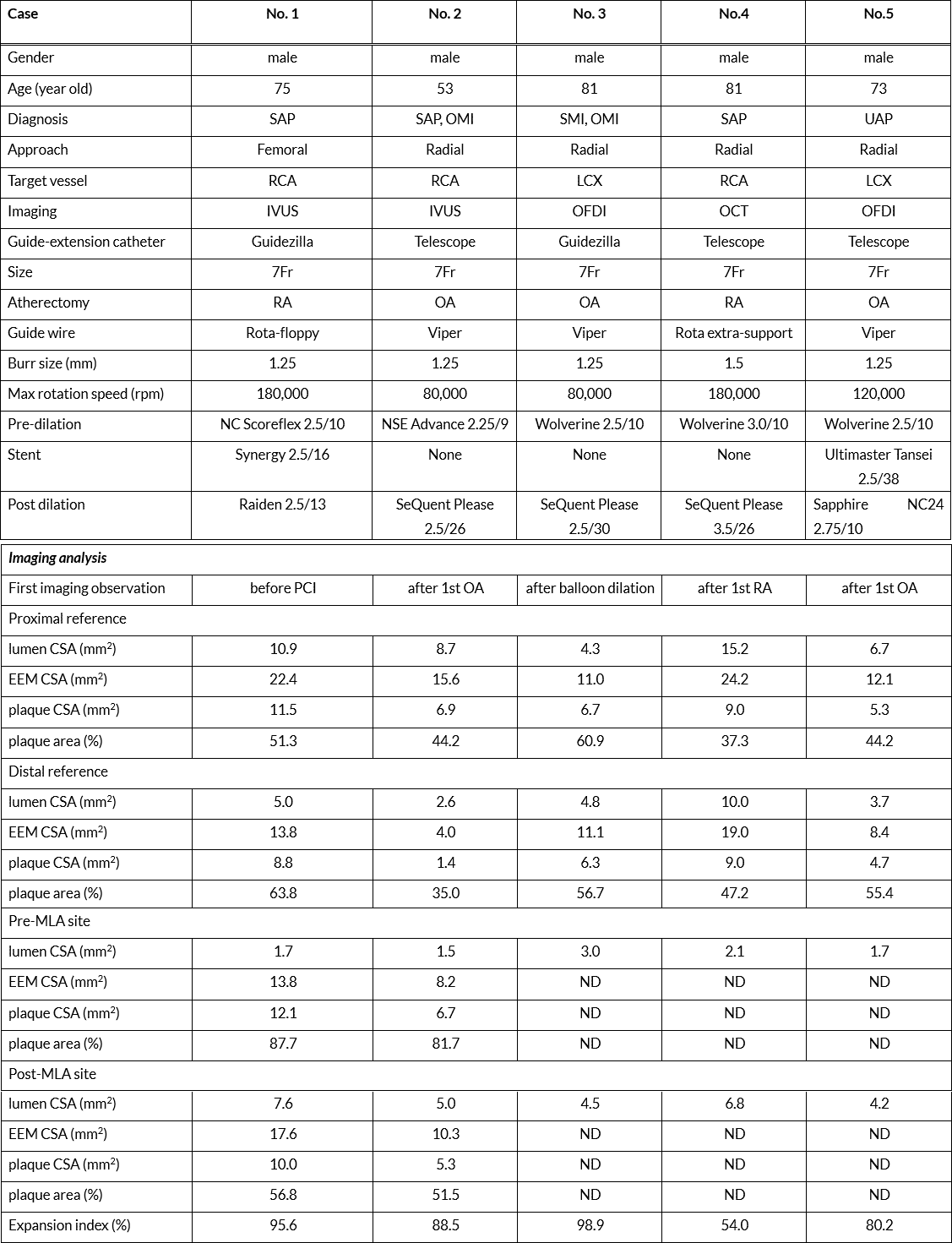
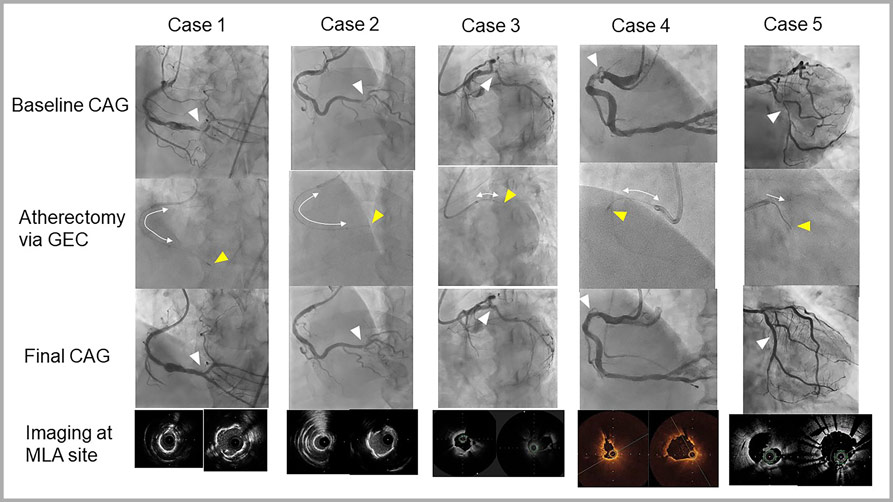
Figure 1. Coronary angiography (CAG) and intravascular imaging of the cases treated with coronary atherectomy via guide extension catheter (GEC) for bending calcified lesions.
Upper panels show baseline CAG, which presented calcified lesions (white triangles). The second panel shows the location of the GEC (white arrows) and burr of the coronary atherectomy (yellow triangle). The third panel shows the final CAG, which presented sufficient luminal dilation in the treated sites (white arrows). The lower panel shows intravascular imaging at the minimum lumen area (MLA) site. Left: first imaging before intervention or after the first session of coronary atherectomy. Right: final image at the same site.
The GEC was advanced as deeply as it could be passed. The length of insertion was more than 20 mm in three cases. The insertion in the ostium of the branch in which the target lesion was located was seen in two cases. The GEC was tightly engaged even during or after the insertion of the atherectomy catheter. Coronary atherectomy was completed through the bending calcified lesion in all cases.
Two cases were treated with the RA system (Rotablator, Boston Scientific, Natick, MA, USA) using a 1.25 mm or 1.5 mm burr, and three cases were treated with the OA system (Diamondback 360, Cardiovascular Systems Inc. St. Paul, MN, USA).
As for the imaging, OCT, OFDI, and IVUS were used in one, two, and two cases, respectively. A 7Fr GEC, Guidezilla (Boston Scientific, Natick, MA, USA) and Telescope (Medtronic, Santa Rosa, CA, USA) were used in two and three cases, respectively.
The treated lesions were in the left circumflex artery in two cases and the right coronary artery (RCA) in three cases.
A scoring balloon was used after coronary atherectomy; Wolverine (Boston Scientific, Natick, MA, USA) in three cases, NSE (Nipro, Osaka, Japan) in one case, and Scoreflex NC (Orbus Neich, Hong Kong, China) in one case.
Three cases were treated with a DCB (SeQuent Please, B. Braun, Berlin, Germany) and the other two cases were treated by deploying a DES in the bending lesion, Synergy (Boston Scientific, Natick, MA, USA) and Ultimaster Tansei (Terumo, Tokyo, Japan).
In the imaging analysis, coronary atherectomy effectively ablated the calcified lesion (Figure 1 and Supplementary Figures 1 and 2), and post-PCI MLA was enlarged to > 5 mm2 in all IVUS cases and > 4.2 mm2 in all OCT/OFDI cases.
Supplementary Figure 1: A representative case of an 81-year-old male treated with rotational atherectomy (RA) combined with a guide extension catheter (GEC) [Case No. 4].
A. Baseline coronary angiography (CAG) showed a severe calcified lesion in the proximal bending port of the right coronary artery (triangles) [Supplementary Movie 2].
B. A 1.5 mm RA burr was unable to advance distally in the severely bending point (arrow).
C. Insertion of a GEC (Telescope, Medtronic) for the strong back-up force (tip at the yellow triangle).
D. Guidewire bias was changed such that it was more along the inner curvature (arrows).
E. RA across the lesion was completed (yellow arrow).
F. Scoring balloon dilation (3.0/10 mm Wolverine, Boston Scientific).
G. Drug-coated balloon (DCB) dilation (3.5/26 mm SeQuent Please, B. Braun).
H. Final CAG [Supplementary Movie 3].
I. Optical coherence tomography (OCT) image at the minimal lumen area site after RA [Supplementary Movie 4].
J. OCT image after using a scoring balloon and subsequent DCB dilation at the same site [Supplementary Movie 5].
![Supplementary Figure 2: A representative case of a 73-year-old male treated with orbital atherectomy combined with a GEC [Case No. 5].](https://www.pcronline.com/var/pcrov3/storage/images/media/pcr/cases/read-and-share-cases/2021/coronary-atherectomy/coronary-atherectomy-calcified-lesion-supp-fig2-case-5/14319014-1-eng-GB/coronary-atherectomy-calcified-lesion-supp-fig2-case-5.jpg)
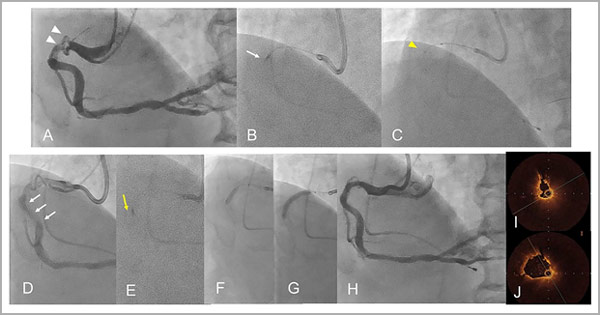
Supplementary Figure 2: A representative case of a 73-year-old male treated with orbital atherectomy (OA) combined with a GEC [Case No. 5].
A. Baseline CAG showed a tortuous true bifurcation lesion in left circumflex artery (triangle) [Supplementary Movie 6].
B. Severe calcification was located lateral to the main branch (arrows). C. An OA burr was advanced distally with the insertion of the GEC (Telescope, Medtronic, arrow).
D. CAG after OA ablation. E. Optical frequency domain imaging (OFDI) after OA at 8,000 rpm showed the ablation according to the wire bias (yellow arrow).
F. OFDI image after OA at 12,000 rpm showed more ablation into the calcified lesion [Supplementary Movie 7]. G. Scoring balloon dilation (2.5/10 mm Wolverine, Boston Scientific) in the main vessel.
H. DCB dilation (2.0/20 mm SeQuent Please, B. Braun) in the side branch.
I. Sirolimus-eluting stent (2.5/28 mm, Ultimaster Tansei, Terumo) implantation.
J. Proximal optimisation technique with a 2.75/10 mm non-compliant balloon. K. Final CAG [Supplementary Movie 8]. L. OFDI image after stent implantation at the same site as in panel E and F [Supplementary Movie 9].
The expansion index ranged from 80.2% to 98.9%, except for one case, which satisfied the previous criteria for optimal lumen expansion7. The remaining one case presented sufficient lumen expansion with an MLA of 6.8 mm2 regardless of the small expansion index (54.0%) due to a large RCA vessel. Changes in wire bias due to insertion of the GEC (Supplementary Figure 1) and favourable wire bias to lesion ablation (Supplementary Figure 2) were also confirmed in the imaging observation.
Discussion
It is challenging to treat calcified bending lesions with coronary intervention due to the following characteristics:
- difficulty in advancement of the device,
- difficulty in performing coronary atherectomy in the calcification, which is likely to be eccentric at the bending site,
- difficulty in the management of guidewire bias for atherectomy,
- and the need for a strong back-up of the guiding catheter for deploying devices.
A GEC inserted into a native coronary artery compensates these requests to obtain strong back-up support4-6, to enable the deployment of a bulky atherectomy device4, 6 and to possibly change the wire bias to favourable sites.
However, there are some limitations of combined use with RA or OA due to the intraluminal size or non-full-jacket construction of the GEC as listed in Table 2.
Table 2: Comparison between rotational and orbital atherectomies in the combination use with a guide-extension catheter (GEC) for coronary bending calcified lesion
Rotational atherectomy | Orbital atherectomy | |
|---|---|---|
Device | Rotablator (Boston Scientific, Natick, MA) | Diamondback 360 (Cardiovascular Systems Inc. St. Paul, MN) |
Device transportation | Resistant | Easier than rotational atherectomy |
Guide catheter system | ≥7Fr | ≥7Fr |
Ability of ablation | 6Fr GEC: Only 1.25mm burr available | 6Fr GEC: Full speck available |
Front head ablation | Possible | Impossible |
Power of ablation | Strong | Not so strong |
Rotational speed | 160,000 – 180,000 rpm | 80,000 rpm Step up to 120,000 rpm only in the case of insufficient calcium ablation identified in the imaging observation. |
Even though a 6Fr GEC is available to insert a 1.25 mm burr in both RA and OA, a 7Fr or larger guiding catheter is strongly recommended to advance the atherectomy catheter without significant friction and to enable the injection of contrast medium via the guiding system. For a 7Fr GEC, a burr of up to 1.5 mm of RA and any burr of OA are available.
The atherectomy catheter should be advanced through the GEC without using the Dyna mode to prevent entrapment of the entry port of the GEC. When significant friction is felt while passing through the entry port, the GEC system should be retrieved. After pre-setting the atherectomy catheter inside the GEC, concomitant advancement of the GEC and atherectomy catheter is recommended.
In terms of lesion crossability, OA is superior to RA due to its flexible front head; however, there remain cases with failed attempts at crossing that require front head ablation to advance the atherectomy catheter thorough the lesion.
The concentric calcification closely distal to the bending lesion is likely to block the OA burr advancement. The stagnated motion of the OA burr in the bending lesion for advancing has a possible risk of enhancing the orbital motion of the burr, resulting in a coronary perforation.
In such cases, change to the RA system is helpful to complete the ablation. Once the OA burr crosses the bending lesion, pulling back the burr slowly through the lesion effectively ablates the eccentric calcification located in the lesser curvature. OA burr rotation speed should be started at 80,000 rpm and stepped up to 120,000 rpm only in the case of insufficient calcium ablation identified in the imaging observation.
The RA burr is more solid and bulky but is feasible for front head ablation. As a stiff guidewire is likely to be located with a bias to the greater curvature in the bending lesion, a careful slow progression of the RA burr is necessary to prevent it from slipping out of the lesion, which may lead to coronary perforation.
When we find excessive guidewire bias to the greater curvature with healthy intima at the bending portion in the imaging observation, the RA should be avoided and pull-back ablation in the OA is suitable to change the guidewire bias to the lesser curvature. We used the RA at recommended rotation speed (180,000 rpm) because there was a risk of more platelet activation at higher speed with stagnation of bur advancement in the bending lesion and more unexpected orbital motion with excessive ablation at lower speed10.
GEC insertion in front of the bending lesion is a possible solution to change the guidewire bias in the bending lesion, as shown in Supplementary Figure 1. Subsequent dilation with a scoring balloon was performed in all cases, which effectively disintegrated the calcium without significant intimal injury1,2,8,9. The GEC is also effective in deploying a bulky scoring balloon in the bent calcified lesion.
Intracoronary imaging effectively assesses calcified coronary lesions in terms of their location, arc, thickness, and longitudinal distribution. OCT/OFDI is superior to IVUS in terms of visualising the calcified borders, precise measurement of the calcium thickness, and effect of lesion modification by atherectomy and/or scoring balloon. Calcium thickness of 500-700 μm or an arc angle of > 230° is the cut-off value for crack formation after balloon dilation, which can provide a larger stent expansion and lesser target lesion revascularisation1-3.
Recently, an OCT-based calcium scoring system has been proposed (maximum calcium angle > 180°, 2 points; maximum calcium thickness > 500 μm, 1 point; calcium length > 5 mm, 1 point), and higher scores indicate less stent expansion and the need for coronary atherectomy3. Although the OCT/OFDI also effectively assesses the bending calcified lesion, softer shaft, and longer tip of an imaging catheter is more challenging to advance through the lesion compared with IVUS.
The combined use of the GEC helps in the safe deployment of the imaging catheter and clear visualisation of the lesion with a smaller amount of flush medium. However, careless flush with deep engagement of the GEC has a risk of increased enlargement of the coronary dissection made by balloon dilation. In the OCT/OFDI assessment after balloon dilation, the coronary arterial pressure at the tip of the GEC should be checked within < 10 mmHg compared with systemic systolic arterial pressure, because pressure damping due to wedge of the catheter to coronary artery varied from 9-94 mmHg11. If the engaged pressure is dumping more, the GEC catheter should be retrieved more proximally to avoid dumping (Supplementary Figure 3).
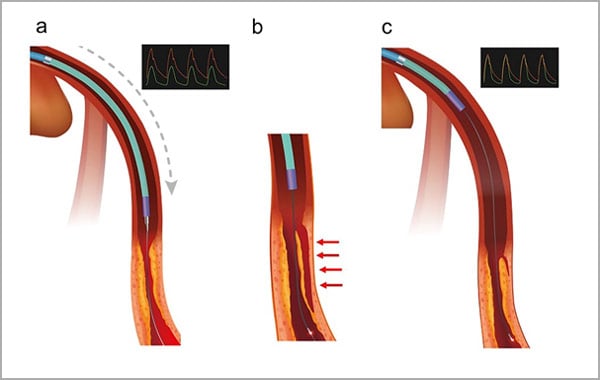
Supplementary Figure 3: Location of the GEC in imaging acquisition of OCT.
(a) Deep engagement of the GEC immediately after balloon dilation in the lesion, which causes intimal dissection. Coronary arterial pressure (green line) is dumping compared with systemic arterial pressure (red line).
(b) Flush to obtain OCT images with the GEC in deep engagement leads to the extension of the dissection (arrows).
(c) The GEC should be retrieved to release the pressure dumping when the flush is performed safely.
Intracoronary imaging is also useful for assessing the wire bias in the bending calcified lesion, which is expected to be the route of the RA/OA burr. As the wire bias is likely to be in the greater curvature of the severely bent lesion, unnecessary coronary atherectomy should be avoided in cases where eccentric calcification is in the lesser curvature at a distance of > 2 mm from the guidewire, in which the burr available for this technique (less than 1.5mm in RA and 1.25mm in OA) is unlikely to contact the calcified lesion. Although differential ablation is expected in both RA and OA, excessive forward pressure into the healthy site is a risk factor for coronary perforation. Deep insertion of the GEC is a possible solution for changing the wire bias under imaging guidance to enable safe completion of coronary atherectomy.
In this series, coronary atherectomy reduced the plaque area from 81.7-87.7% to 51.5-56.8%. However, in case numbers 2, 3, and 4, eccentric calcified lesions remained even after coronary atherectomy and were treated with a DCB after dilation using a scoring balloon. The intravascular lumen was dilated sufficiently (4.5-6.8 mm2) to avoid DES implantation in the bending calcified lesion, where stent fracture is likely to occur12. The recurrent appearance of calcified nodules in the already treated site is also likely to occur, and stent-less treatment with a DCB is reasonable.
Limitations
This was a retrospective, observational study that included a small number of initial cases. Long-term follow-up was not available.
Conclusion
Coronary atherectomy, in combination with a GEC for bending calcified lesions, is effective in ablating calcified lesions under imaging guidance.
Impact on daily practice
Coronary atherectomy for bending calcified coronary lesions is challenging, and the combined use of a GEC helps in advancing a bulky atherectomy device and for managing guidewire bias in the calcified lesion. It enables complete imaging-guidance and large dilation using a scoring balloon.
Funding statement
None.
Conflict of Interest
The authors do not have any conflict of interest.
- Kubo T, Shimamura K, Ino Y, Yamaguchi T, Matsuo Y, Shiono Y, Taruya A, Nishiguchi T, Shimokado A, Teraguchi I, Orii M, Yamano T, Tanimoto T, Kitabata H, Hirata K, Tanaka A and Akasaka T. Superficial Calcium Fracture After PCI as Assessed by OCT. JACC Cardiovasc Imaging. 2015;8:1228-9.
- Maejima N, Hibi K, Saka K, Akiyama E, Konishi M, Endo M, Iwahashi N, Tsukahara K, Kosuge M, Ebina T, Umemura S and Kimura K. Relationship Between Thickness of Calcium on Optical Coherence Tomography and Crack Formation After Balloon Dilatation in Calcified Plaque Requiring Rotational Atherectomy. Circ J. 2016;80:1413-9.
- Fujino A, Mintz GS, Matsumura M, Lee T, Kim SY, Hoshino M, Usui E, Yonetsu T, Haag ES, Shlofmitz RA, Kakuta T and Maehara A. A new optical coherence tomography-based calcium scoring system to predict stent underexpansion. EuroIntervention. 2018;13:e2182-e2189.
- Kobayashi N, Yamawaki M, Hirano K, Araki M, Sakai T, Sakamoto Y, Mori S, Tsutsumi M, Nauchi M, Sahara N, Honda Y, Makino K, Shirai S, Mizusawa M, Sugizaki Y, Nakano T, Fukagawa T, Kishida T, Kozai Y, Setonaga Y, Goda S and Ito Y. Use of the orbital atherectomy system backed up with the guide-extension catheter for a severely tortuous calcified coronary lesion. SAGE Open Med Case Rep. 2020;8:2050313X20921081.
- Sakakura K, Taniguchi Y, Tsukui T, Yamamoto K, Momomura SI and Fujita H. Successful Removal of an Entrapped Rotational Atherectomy Burr Using a Soft Guide Extension Catheter. JACC Cardiovasc Interv. 2017;10:e227-e229.
- Pellicano M, Flore V, Barbato E and De Bruyne B. From debulking to delivery: sequential use of rotational atherectomy and Guidezilla for complex saphenous vein grafts intervention. BMC Cardiovasc Disord. 2018;18:122.
- Belguidoum S, Meneveau N, Motreff P, Ohlman P, Boussaada M, Silvain J, Guillon B, Descotes-Genon V, Lefrancois Y, Morel O and Amabile N. Relationship between stent expansion and post-PCI fractional flow reserve: a DOCTORS sub study. EuroIntervention. 2020.
- Li Q, He Y, Chen L and Chen M. Intensive plaque modification with rotational atherectomy and cutting balloon before drug-eluting stent implantation for patients with severely calcified coronary lesions: a pilot clinical study. BMC Cardiovasc Disord. 2016;16.
- Furuichi S, Tobaru T, Asano R, Watanabe Y, Takamisawa I, Seki A, Sumiyoshi T and Tomoike H. Rotational atherectomy followed by cutting-balloon plaque modification for drug-eluting stent implantation in calcified coronary lesions. J Invasive Cardiol. 2012;24:191-5.
- Yamamoto T, Yada S, Matsuda Y, Otani H, Yoshikawa S, Sasaoka T, Hatano Y, Umemoto T, Ueshima D, Maejima Y, Hirao K, Ashikaga T. A Novel Rotablator Technique (Low-Speed following High-Speed Rotational Atherectomy) Can Achieve Larger Lumen Gain: Evaluation Using Optical Frequency Domain Imaging. J Interv Cardiol. 2019 May 20;2019:9282876. doi: 10.1155/2019/9282876. eCollection 2019.
- Pacold I, Hwang MH, Piao ZE, Scanlon PJ, Loeb HS. The mechanism and significance of ventricularization of intracoronary pressure during coronary angiography. Am Heart J. 1989 ;118:1160-6.
- Nagai T, Mizobuchi M, Funatsu A, Kobayashi T and Nakamura S. Acute and mid-term outcomes of drug-coated balloon following rotational atherectomy. Cardiovasc Interv Ther. 2020;35:242-249.




1 comment
This presentation shows extent of advanced interventional and imaging techniques being used now a days to deal with complex calcified case of PCI!