First report of outcomes in the TRILUMINATE pivotal clinical trial in patients with severe tricuspid regurgitation
Reported from TCT 2022
Alex Sticchi provides his take on the TRILUMINATE pivotal trial which was presented by David H. Adams at the TCT Congress 2022.
PICOT analysis of TRILUMINATE. Courtesy of Alex Sticchi @Sticchi_Alex. Source: PCRonline.com
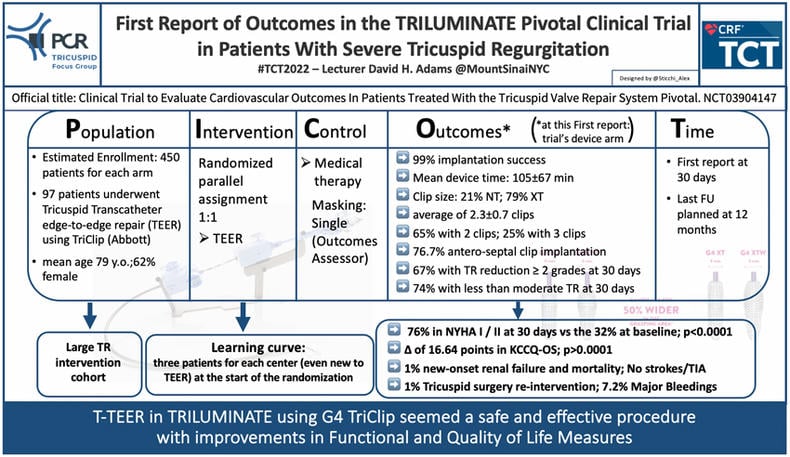
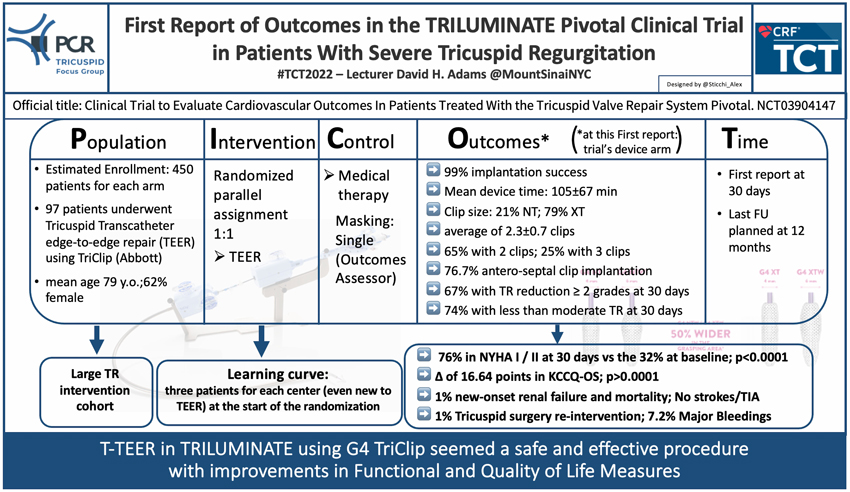
TCT 2022 brought us exciting and confirmatory results on Tricuspid intervention. The TRILUMINATE Pivotal trial (Clinical Trial to Evaluate Cardiovascular Outcomes In Patients Treated With the Tricuspid Valve Repair System Pivotal. ClinicalTrials.gov Identifier: NCT03904147) has been presented by Prof David H. Adams (Mount Sinai NYC) showing important results for the future treatment of Tricuspid regurgitation (TR). The TRILUMINATE Pivotal trial is a randomized interventional trial comparing the TriClip G4 Device (Abbott) versus medical therapy evaluating:
- Change in TR
- Freedom from major adverse events occurring after the index procedure
- Change in quality of Life through assessment of Kansas City Cardiomyopathy Questionnaire (KCCQ) Score
- Change in 6 Minute Walk Test (6MWT)
The Primary Endpoint is a hierarchical composite of all-cause mortality or tricuspid valve surgery, rate of heart failure hospitalizations, and assessment of the quality of life improvement using the KCCQ.
The study plan to enrol 450 patients for each arm with a 1:1 randomization to intervention or medical therapy and a follow-up of 12 months.
One interesting feature of this trial is the enrolling of three patients for each center at the start of the randomization, even centers without previous experience in Tricuspid Transcatheter edge-to-edge repair (T-TEER). This allows capturing the learning curve during the ongoing trial. This insight represents a peculiarity of this trial, and it has never been systematically investigated. Specifically, the learning curve represents an important issue not only for the procedural technique but also for the pre-procedural planning and patient preparation.
The report presented at the TCT 2022 showed the 30-day results of 97 patients enrolled in the interventional arm. The population presented a mean age of 79 years, and 62% of patients were women. 91% of the population had a functional disease, with 61% presenting massive or torrential TR. 68% was in class NYHA III/IV.
In terms of efficacy, TriClip was successfully implanted in 99% of cases, in a mean device time of 105±67 minutes, and with a rate of 2.3±0.7 clips per patient (65% implanted two clips). In addition, in 79% of cases, the operators implanted a long clip (XT), most in the antero-septal position (76.7%).
At 30 days, the TR severity was inferior or equal to moderate in 74% of patients, with a reduction of at least two grades of disease in 67% of the population and of at least 1 grade in 91%.
Contemporary, this first report presented a significant improvement in both function and quality of life with 74% of patients in NYHA class I-II from the 32% at baseline (p<0.0001) and a favourable gain of 16.64 points (p<0.0001) in KCCQ.
Regarding safety, the 30-day report showed 1% of cardiovascular mortality and new renal failure, no stroke or TIA, and 1% of re-intervention for Tricuspid valve surgery. Seven patients (7.6%) presented major bleeding, 5 with gastro-intestinal ones.
In summary, the first report at 30 days of the TRILUMINATE trial confirmed the safety and efficacy of the T-TEER using the TriClip G4 device with functional and quality of life improvement.
The design of this exciting study will help us to understand more about this treatment and patient selection.





No comments yet!