Dapagliflozin in acute myocardial infarction: Cardiometabolic benefits without impact on cardiovascular death or heart failure hospitalization: The jury is still out
Reported from AHA 2023
Luis Ortega Paz provides his take on DAPA-MI - A registry-based randomized trial of dapagliflozin in patient with acute myocardial infarction without diabetes, which was presented by Stefan James at AHA 2023 in Philadelphia.
Why this study – the rationale/objective?
The DAPA-MI trial aimed to assess the efficacy of dapagliflozin in patients with acute myocardial infarction (MI) and impaired left ventricular function who did not have prior diabetes or chronic heart failure. The rationale was to explore therapies that could reduce the risk of adverse cardiovascular and metabolic outcomes in this patient population.(1)
How was it executed – the methodology?
The trial was an international registry-based, randomized, double-blind, placebo-controlled trial conducted in Sweden and the United Kingdom. Patients were randomly assigned to receive dapagliflozin 10mg/qd (n=2019) or matching placebo (n=1998). The primary outcome was a hierarchical composite of death, hospitalization for heart failure, nonfatal MI, atrial fibrillation/flutter, type 2 diabetes mellitus, and other criteria, analyzed using the win ratio method.
What is the main result?
The primary outcome showed significantly more 'wins' for dapagliflozin than for placebo (win ratio, 1.34; 95% CI, 1.20 to 1.50; P<0.001), mainly driven by added cardiometabolic outcomes (Figure).
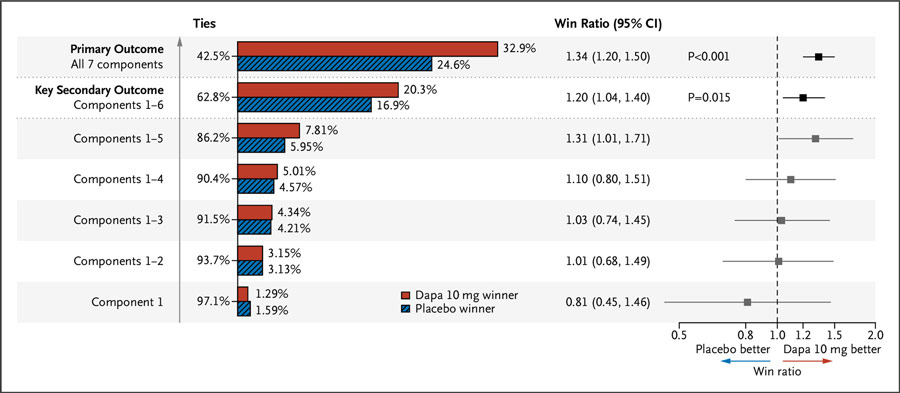
Figure: Primary and key secondary hierarchical composite outcome, assessed by the win ratio method.
Source: NEJM Evidence
The composite of time to cardiovascular death/hospitalization for heart failure occurred similarly in both groups. Dapagliflozin also led to a lower incidence of new diagnosis of type 2 diabetes and a reduced mean body weight.(2)
Critical reading and the relevance for clinical practice
This trial indicates that dapagliflozin can offer significant cardiometabolic benefits in patients hospitalized for acute MI with impaired left ventricular function without prior diabetes or chronic heart failure. However, compared with placebo, it had no significant impact on the composite of cardiovascular death or hospitalization for heart failure. Using a registry-based approach, the trial design allowed for efficient data collection and high enrollment. Despite the positive outcomes, the trial faced limitations, including the unexpectedly low event rate for cardiovascular death and hospitalization for heart failure, leading to a change in the primary outcome and analysis plan. The trial's results primarily reflect benefits in cardiometabolic outcomes, with limited data on individual or composite cardiovascular events.
PARADISE-MI trial(3) already showed that even for these fantastic heart failure drugs, post-MI patients are a challenging population to show any additional clinical benefit. The ongoing EMPACT-MI trial(4) will compare the role of empagliflozin versus placebo in a similar population. The jury is still out.
References
- James S, Erlinge D, Storey RF et al. Rationale and design of the DAPA-MI trial: Dapagliflozin in patients without diabetes mellitus with acute myocardial infarction. Am Heart J 2023.
- James S, Erlinge D, Storey RF et al. Dapagliflozin in Myocardial Infarction without Diabetes or Heart Failure. NEJM Evidence;0:EVIDoa2300286.
- Pfeffer MA, Claggett B, Lewis EF et al. Angiotensin Receptor-Neprilysin Inhibition in Acute Myocardial Infarction. N Engl J Med 2021;385:1845-1855.
- Harrington J, Udell JA, Jones WS et al. Empagliflozin in patients post myocardial infarction rationale and design of the EMPACT-MI trial. Am Heart J 2022;253:86-98.





No comments yet!