Three-year results of HOST-POLYTECH-ACS RCT comparing durable- vs. degradable-polymer DES
Reported from ESC Congress 2023
Alex Sticchi provides his take on the 3-year results of HOST-POLYTECH-ACS RCT which were presented by Hyo-Soo Kim during the ESC 2023 congress in Amsterdam.
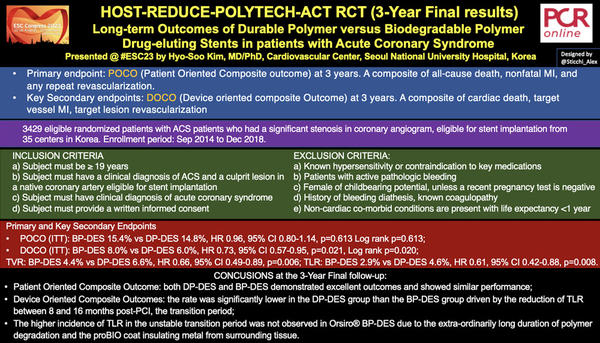
HOST-REDUCE-POLYTECH RCT (3-Year Final results) - Long-term Outcomes of Durable Polymer versus Biodegradable Polymer Drug-eluting Stents in patients with Acute Coronary Syndrome - Designed by @Sticchi_Alex
The Harmonizing Optimal Strategy for Treatment of Coronary Artery Diseases - Comparison of Reduction of Prasugrel Dose or Polymer Technology in ACS Patients (HOST-REDUCE-POLYTECH-ACS) trial has randomized 3429 ACS patients who had a significant stenosis in coronary angiogram, eligible for stent implantation in 35 centers.
Why this study – the rationale/objective?
Drug-eluting stents (DES) are currently considered the gold standard for patients undergoing percutaneous coronary intervention (PCI) with a residual rate of adverse events around 2-3% per year. This continues to move forward the progression of stent technologies, one of which is the use of biodegradable polymers in order to reduce adverse events. The biodegradable polymer (BP-DES) has the potential advantage, after drug elution, of degrading leaving behind a bare metal stent with the reduction of local inflammatory reaction and consequent increased risk of stent compared to a durable polymer (DP-DES). To date, there is limited evidence comparing DP-DES and BP-DES in patients undergoing Acute Coronary Syndrome (ACS) where there is high vascular inflammation.
Preliminary one-year result of the HOST-REDUCE-POLYTECH-ACS trial showed:
- DP-DES was comparable to BP-DES in patient-oriented composite outcomes (POCO)
- DP-DES was better than BP-DES in device-oriented composite outcomes (DOCO)
Long-term follow-up is required to evaluate the influence of polymer technologies considering that the duration of polymer degradation in BP-DES varies from 4 to 15 months after PCI.
How was it executed? - the methodology
The study is a multi-centre (35 centres in Korea) randomized study conducted from September 2014 to December 2018.
ACS patients who had significant stenosis in coronary angiogram, eligible for PCI, have been randomized 1:1 to the implantation of DP-DES or BP-DES. The use of intracoronary imaging was left to the discretion of the operator.
Inclusion criteria:
- a) Subject must be ≥ 19 years
- b) Subject must have a clinical diagnosis of ACS and a culprit lesion in a native coronary artery eligible for stent implantation
- c) Subject must have clinical diagnosis of acute coronary syndrome
- d) Subject must provide a written informed consent
Exclusion Criteria:
- a) Known hypersensitivity or contraindication to key medications
- b) Patients with active pathologic bleeding
- c) Female of childbearing potential, unless a recent pregnancy test is negative
- d) History of bleeding diathesis, known coagulopathy
- e) Non-cardiac co-morbid conditions are present with life expectancy <1 year
The primary endpoint was a Patient-Oriented Composite Outcome (POCO) at 3 years of all-cause death, nonfatal MI, and any repeat revascularization.
The key secondary endpoint was a Device-Oriented Composite Outcome (DOCO) at 3 years of cardiac death, target vessel MI, target lesion revascularization.
What is the main result?
The study randomized 3429 patients, 1713 to DP-DES and 1700 to BP-DES. The 3-year follow-up covered 96% of patients.
Baseline clinical characteristics were similar between the two groups with age of 63.0±11.1 in DP-DES and 63.1±11.1 in BP-DES. In both groups, males represented around 78% of patients. The diabetics were 46.1% in DP-DES and 43.9% in BP-DES. Moreover, the rates of ST elevation myocardial infarction (STEMI), Non STEMI, and unstable angina were similar between the groups.
Multivessel disease was around 54% in both groups. The IVUS use was 29.9% in DP-DES and 32.2% in BP-DES arm, with a mean around 1.4 lesion number per person, around 1.7 stent number per person in both groups, with more than 40 mm in both groups.
Primary and key secondary endpoints:
- POCO (ITT): BP-DES 15.4% vs DP-DES 14.8%, HR 0.96, 95% CI 0.80-1.14, p=0.613, Log rank p=0.613;
- DOCO (ITT): BP-DES 8.0% vs DP-DES 6.0%, HR 0.73, 95% CI 0.57-0.95, p=0.021, Log rank p=0.020;
A following analysis about Thin-Struts DES showed the persistence of significant differences in DOCO in favor of DP-DES:
POCO: BP-DES 16.4% vs DP-DES 14.8%, HR 0.89, 95% CI 0.73-1.09, p=0.255, Log rank p=0.257;
DOCO: BP-DES 8.0% vs DP-DES 6.0%, HR 0.73, 95% CI 0.55-0.99, p=0.040, Log rank p=0.041.
From the Kaplan-Meyer curves (KM), the investigators found the need for a landmark analysis according to time of polymer degradation. This analysis showed no difference in this transition period in POCO but a significant one in DOPO, exactly in that time period from 240 to 480 days from the procedure, BP-DES 3.3% vs DP-DES 1.8%, HR 0.54, 95% CI 0.34-0.849, p=0.007. No differences after 480 days.
The difference was driven by
- Target Vessel Revascularization (TVR): BP-DES 4.4% vs DP-DES 6.6%, HR 0.66, 95% CI 0.49-0.89, p=0.006;
- Target Lesion Revascularization (TLR): BP-DES 2.9% vs DP-DES 4.6%, HR 0.61, 95% CI 0.42-0.88, p=0.008, Log rank p=0.007.
The analysis of the four BP-DES (Biomatrix/Nobori, Ultimaster, Synergy, Orsiro) along three periods (Early up to 240 days; Transition period from 240 to 480 days; and Late period beyond 480 days) highlighted the low rate of TLR of Orsiro (Biotronik AG, Bülach, Switzerland) in the Transition period with numerically higher rate for the other platforms. Furthermore, the total 3-year TLR rate of Orsiro was a little bit lower than the other DES.
So, the authors investigated the KM of the Orsiro compared to DP-DES (3.2% vs 2.9%, respectively) showing no differences in TLR at 3 years (HR 0.63, 95% CI 0.35-1.16, p=0.137, Log rank p=0.674), but still a significant difference in the transition period (Orsiro 1.1% vs DP-DES 1.0%, HR 0.20, 95% CI 0.10-0.78, p=0.015).
Critical reading and the relevance for clinical practice
The results of this interesting study showed that in ACS patients DP-DES and BP-DES had similar outcomes in terms POCO at 3 years but DOCO were significantly lower in the DP-DES group.
This was mainly driven by the reduction of TLR between 240 and 480 days (8-16 months) post-PCI. This so-called transition period, when the vessel responds to polymer degradation and subsequent bare metal exposure of BP-DES, was not observed only in the Orsiro® BP-DES. This favourable outcome could be associated with the very long duration of the polymer degradation, slow but consistent, and the proBIO coat insulating metal from surrounding tissue.
Multiple factors could have influenced the outcomes after DES implantation, especially technical factors related to stent deployment. Lesion characteristics and medical therapy are other factors to consider too.
The study has several limitations. First, the study was not adequately powered to assess the individual components of the composite endpoint, let alone rare device-oriented clinical events, such as stent thrombosis. Secondly, the study was not double-blinded, investigators acknowledged which arm the patient was enrolled in.
Finally, this investigation showed the safety and efficacy of BP-DES in ACS, highlighting the potential benefit of specific platform characteristics. Still, further randomized data is required besides device progress to improve the rate of safety and efficacy in ACS treatment.






No comments yet!