Intravascular ultrasound-guided versus angiography-guided percutaneous coronary intervention in acute coronary syndromes: The multicenter, randomized, blinded, IVUS-ACS Trial
Reported from ACC.24
Marco Spagnolo and Daniele Giacoppo report the main results of the IVUS-ACS trial presented by Shao-Liang Chen, from the Nanjing First Hospital, Nanjing Medical University, Nanjing, in a Featured Clinical Research session at ACC.24 in Atlanta and simultaneously published in The Lancet.(1)
Courtesy of Marco Spagnolo and Daniele Giacoppo - Source: PCRonline.com
Why this study? – the rationale/objective
Randomized clinical trials and meta-analyses have consistently demonstrated that intravascular ultrasound (IVUS) for guiding percutaneous coronary intervention (PCI) reduces cardiovascular outcomes compared to coronary angiography alone.(2-4) However, most of the available high-quality evidence about IVUS-guided PCI refers to the treatment of chronic stable lesions.(2-4)
As many patients with acute coronary syndrome (ACS) exhibit a variable thrombotic burden upon eroded or disrupted lipid-rich coronary plaques, the role of IVUS during PCI may be prognostically significant. Indeed, IVUS provides improved plaque characterization, mechanistic insights, and guidance in stenting optimization. Nevertheless, it could be also argued that IVUS might yield limited additional information for evident culprit disease, delay procedural times, and face acute lesion constraints in guiding stenting optimization.
To date, only three small randomized trials have focused on intravascular imaging-guided PCI in patients with ACS, providing mixed results and primarily comparing optical coherence tomography (OCT) guidance with angiography alone.(5-7) Against this background, the IVUS-ACS trial aimed to define whether IVUS guidance improves long-term cardiovascular outcomes compared with coronary angiography alone after PCI for the treatment of ACS.
How was it executed? – the methodology
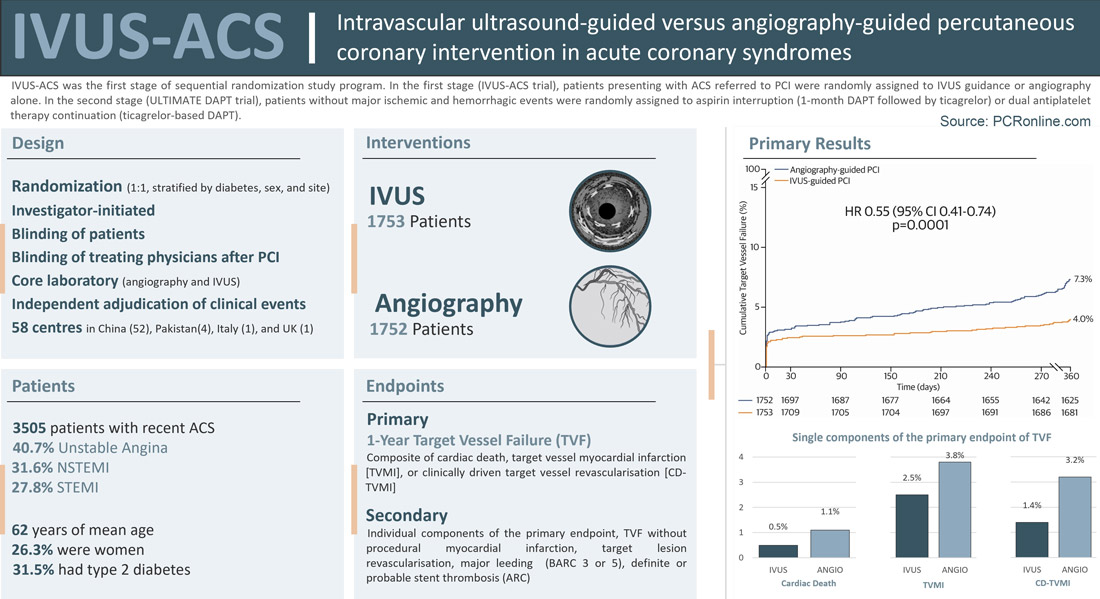
The IVUS-ACS trial was an international, investigator-initiated, randomized trial of patients presenting with ACS referred to PCI at 58 centres, predominantly sited in China.(1)
The trial was the first stage of a sequential randomization study program including another trial (ULTIMATE-DAPT) designed to assess different antiplatelet strategies following PCI.(8) In the first stage (IVUS-ACS), patients were randomized to IVUS- or angiography-guided PCI. The randomization sequence was stratified by diabetes status, sex, and site using dynamic minimization. In the second stage (ULTIMATE-DAPT), patients enrolled in IVUS-ACS who did not experience major ischemic and haemorrhagic events within 30 days post-PCI were randomly assigned to aspirin interruption (i.e., 1 month of dual antiplatelet therapy [DAPT] followed by ticagrelor monotherapy) or DAPT continuation.
Patients were eligible for inclusion in the IVUS-ACS trial if they had experienced an ACS within the preceding 30 days, a de novo culprit lesion in a native coronary segment could be identified by coronary angiography, and there was an indication for PCI with second-generation drug-eluting stents. Key exclusion criteria included prior coronary artery bypass grafting, prior intracranial bleeding or disease, recent ischemic cerebrovascular accident, severe chronic kidney disease, severe platelet count reduction, planned surgery, contraindications to antiplatelet therapy, need for oral anticoagulation, and limited life expectancy. While physicians and staff in the cardiac catheterization laboratory were necessarily unblinded to the randomization, all personnel interacting with patients after PCI were blinded to treatment allocation.
The primary endpoint was 1-year target-vessel failure TVF, a composite of cardiac death, target-vessel myocardial infarction, or clinically driven target-vessel revascularization. Secondary endpoints included the individual components of the primary endpoint, target vessel failure without procedural myocardial infarction, target lesion revascularization, Bleeding Academic Research Consortium types 3 or 5 bleeding, and definite or probable stent thrombosis. Optimal IVUS-guided PCI criteria for non-left main lesions (ULTIMATE criteria) included a minimum stent area >5.0 mm2 or >90% of the minimum lumen area at the distal reference segment, a plaque burden <55% within 5 mm proximal or distal to the stent edge, and the absence of medial dissection >3 mm in length. Optimal IVUS-guided PCI criteria for left main lesions (EXCEL criteria) included a minimum stent area >10 mm2 for the left main segment, >7 mm2 for the ostial/proximal left anterior descending, and >6 mm2 for the ostial/proximal left circumflex. Clinical events were independently adjudicated. Angiograms and IVUS before and after PCI procedures were analysed by independent core laboratories.
Assuming a 1-year TVF incidence of 10% associated with coronary angiography and a common dropout rate of 5%, it was estimated that a sample size of 3486 patients was required to detect with a power of 80% a relative risk reduction of 28% associated with IVUS at a 2-sided p value of 0.05.
What is the main result?
From August 2019 to October 2022, a total of 3,505 patients were randomly assigned to IVUS-guided PCI (n=1753) or angiography-guided PCI (n=1752). Most of them (96.5%) were recruited upon presentation to the emergency room. The baseline clinical and angiographic characteristics were well-balanced between the two groups. The median age was 62 years [54-69], 921 patients (26.3%) were female, and 1105 (31.5%) of patients had type 2 diabetes. The clinical presentation was unstable angina in 1425 patients (40.7%), non-ST-segment elevation myocardial infarction (STEMI) in 1107 (31.6%), and ST-segment elevation myocardial infarction in 973 (27.8%). Left main and left anterior descending were the target vessels in 4.3% and 56.5%, respectively. The target lesion involved a true major bifurcation in 15.3%, diseased segments >30 mm in 72.5%, moderate-to-severe calcification in 7.7%, and thrombus in 9.0%.
IVUS was not used for 10 (0.6%) of the patients assigned to IVUS guidance for technical reasons. Among the 1743 patients (99.4%) who received IVUS as randomized, 1737 patients (99.7%) had pre-PCI assessment and 1743 patients (100.0%) had post-PCI assessment. In the angiography group, 9 (0.5%) patients received pre-PCI IVUS, but no patients had post-PCI IVUS. Patients assigned to IVUS-guided PCI received longer stents (33 mm [24-52] vs 30 [23-47; p<0.0001), achieved larger stent diameters (3.20 ± 0.44 mm vs 3.12 ± 0.45; p<0.0001), and underwent more frequently post-dilation (96.9% vs. 93.2%; p<0.0001) compared with those assigned to angiography-guided PCI.
After PCI completion, IVUS-guided PCI was associated with higher minimum lumen diameter (2.76 mm [2.38-3.17] vs 2.70 mm [2.34-3.04]; p=0.0007) and percentage diameter stenosis (12.9% [6.5-19.3] vs. 14.0% [7.9-20.3]; p=0.0005) compared with angiography-guided PCI. Angiographic complete revascularization and periprocedural myocardial infarction (1.9% vs. 2.4%, p=0.28) did not significantly differ between groups. Administered contrast media volume (150 mL [130-190] vs 150 mL [120-180]; p<0.0001) and procedure duration (50 min [35-70] vs 30 mL [20-48]; p<0.0001) were higher with IVUS guidance compared to angiography guidance. A total of 254 patients (7.2%) required an additional staged revascularization.
Thirty days after PCI, 1713 patients (97.7%) in the IVUS-guided PCI group and 1687 patients in the angiography-guided PCI group (96.3%) underwent a second randomisation (ULTIMATE-DAPT trial) to ticagrelor monotherapy (IVUS-guided PCI: 856 patients, 50.0%; 844 patients, 50.0%) or DAPT (IVUS-guided PCI: 857 patients, 50.0%; angiography-guided group: 843 patients, 50.0%).
At 1 year, the primary composite endpoint of TVF occurred less frequently in the IVUS-guided PCI group than in the angiography-guided PCI group (4.0% vs. 7.3%; hazard ratio [HR] 0.55, 95% confidence interval [CI] 0.41–0.74, p=0.0001). In the IVUS-guided PCI group, TVF was 3.2% in patients who met optimal stent implantation criteria in the IVUS-guided PCI group and 7.1% in patients who did not meet optimal stent implantation criteria. This benefit was associated with significant reductions in the individual endpoint of target vessel myocardial infarction (2.5% vs. 3.8%; HR 0.63, 95% CI 0.43-0.92, p=0.018) and clinically driven target vessel revascularization (1.4% vs. 3.2%; HR 0.44, 95% CI 0.27-0.72) in the IVUS-guided PCI group compared with the angiography-guided PCI group, while cardiac death did not significantly differ between groups (0.5% vs. 1.1%; HR 0.56, 95% CI 0.24-1.29, p=0.17). The target vessel myocardial infarction was essentially driven by reduced non-procedural myocardial infarction in the IVUS-guided PCI group compared with the angiography-guided PCI group (0.6% vs. 1.5%; HR 0.41, 95% CI 0.20-0.84, p=0.014). Clinically driven target lesion revascularization (1.3% vs. 2.5%; HR 0.52, 95% CI 0.31-0.88, p=0.014) and definite or probable stent thrombosis (0.6% vs. 0.9%; HR 0.82, 95% 0.35-1.90; p=0.64) were significantly lower in the IVUS-guided PCI group compared with the angiography-guided PCI group.
TVF between groups remained consistent across major subgroups, including diabetes (presence: HR 0.49, 95% CI 0.28-0.83; absence: HR 0.59, 95% CI 0.41-0.84; Pinteraction=0.48), index ACS variant (unstable angina: HR 0.55, 95% CI 0.32-0.92; NSTEMI: 0.61, 95% 0.35-1.08; STEMI: 0.54, 95% CI 0.34-0.85; Pinteraction=0.26), multivessel disease (presence: HR 0.46, 95% CI 0.29-0.75; absence: HR 0.62, 95% CI 0.43-0.89; Pinteraction=0.58), and antiplatelet therapy (DAPT: HR 0.60, 95% CI 0.39-0.93; ticagrelor monotherapy: HR 0.46, 95% CI 0.30-0.72; Pinteraction=0.48). In addition, TVF between groups remained consistent in the per-protocol analysis.
Critical reading and the relevance for clinical practice
IVUS-ACS is the first randomized clinical trial comparing IVUS-guided PCI vs angiography-guided PCI exclusively in patients with ACS. The main conclusions of IVUS-ACS indicate that using IVUS to guide and optimize the implantation of drug-eluting stents significantly reduces the risk of TVF at 1 year compared with angiography alone. This reduction is largely attributable to reductions in target vessel myocardial infarctions, particularly non-periprocedural, and target vessel and lesion repeat revascularization. Despite IVUS use was associated with increased contrast media administration and longer procedural times compared with angiography alone, there were no differences in terms of safety outcomes at short and long-term follow-up.
The IVUS-ACS trial results generally align with previous studies conducted on patients with chronic coronary syndrome, reinforcing the notion that when compared with coronary angiography, IVUS is associated with improved hard clinical endpoints. Importantly, the remarkable differences in non-procedural target vessel myocardial infarction and target lesion revascularization observed between IVUS- and angiography-guided PCI groups support a direct link between the guidance strategy and target lesion. Notably, the benefit of IVUS guidance compared with angiography alone in terms of 1-year TVF was essentially observed among patients who met the prespecified IVUS criteria for optimal stent implantation.
Interestingly, there was no significant interaction between the type of guidance (IVUS vs. angiography alone) and the antiplatelet strategy (12-month ticagrelor-based DAPT vs. 1-month ticagrelor-based DAPT followed by ticagrelor monotherapy). Although this analysis is exploratory as the trial did not have statistical power to test the interaction, 1-month DAPT followed by ticagrelor monotherapy did not result in significantly different 1-year ischemic outcomes compared with 12-month ticagrelor-based DAPT, regardless of the type of guidance employed during the index PCI.
Some considerations are required when interpreting the results of the IVUS-ACS trial.
- Firstly, the operators were not blinded to the guidance strategy, potentially introducing performance bias. Nonetheless, while masking the guidance strategy is technically unfeasible, standardized criteria to define optimal stent implantation were employed and treating physicians outside the catheterization laboratory were unaware of the treatment assignment.
- Secondly, the results may not be generalizable to any pattern of coronary artery disease since multivessel and complex coronary artery diseases were not particularly represented. Although it is plausible to expect higher benefits from IVUS guidance compared with angiography alone in more complex coronary artery disease patterns, available data mostly refer to chronic coronary artery disease and the implications of systematic use of IVUS for plaques associated with large thrombotic burden warrants more analysis.
- Thirdly, the expertise of operators may be pivotal in reproducing the outcomes observed in the trial. Indeed, the IVUS-ACS participating centres were selected based on the PCI volume and the operators routinely employed IVUS-guidance during PCI.
- Fourthly, IVUS guidance benefits should be weighed against the drawbacks of an increased contrast media volume administration and prolonged procedural times. These drawbacks may be particularly relevant for patients suffering from severe chronic kidney disease and those presenting with hemodynamic instability. The benefit of IVUS guidance may be offset by the implications of higher contrast media volume administration and prolonged procedural times in patients suffering from severe chronic kidney disease and presenting with hemodynamic instability.
- Fifthly, the lower-than-expected incidence of TVF in the angiography-guided group implies a reduced statistical power for the primary endpoint. Finally, the diagnostic approach for procedural myocardial infarction, based on clinical suspicion, may have introduced a degree of uncertainty to the event rates, despite efforts to minimize bias. Nonetheless, non-periprocedural myocardial infarction was significantly reduced in patients assigned to IVUS-guided PCI compared with those assigned to angiography-guided PCI.
In conclusion, although ongoing research (e.g., NCT04775914, NCT05007535) will enrich available evidence on IVUS guidance for PCI, the IVUS-ACS trial results support a broader use of IVUS during PCI for ACS.
REFERENCES
- Ge Z, Kan J, Gao X, Raza A. Ticagrelor alone versus ticagrelor plus aspirin from month 1 to month 12 after percutaneous coronary intervention in patients with acute coronary syndromes (ULTIMATE-DAPT): a randomised, placebo-controlled, double-blind clinical trial. Lancet 2024;(Ahead of Print).
- Giacoppo D, Laudani C, Occhipinti G et al. Coronary Angiography, Intravascular Ultrasound, and Optical Coherence Tomography in the Guidance of Percutaneous Coronary Intervention: A Systematic Review and Network Meta-Analysis. Circulation 2024.
- Zhang J, Gao X, Kan J et al. Intravascular Ultrasound Versus Angiography-Guided Drug-Eluting Stent Implantation: The ULTIMATE Trial. J Am Coll Cardiol 2018;72:3126-3137.
- Hong SJ, Kim BK, Shin DH et al. Effect of Intravascular Ultrasound-Guided vs Angiography-Guided Everolimus-Eluting Stent Implantation: The IVUS-XPL Randomized Clinical Trial. JAMA 2015;314:2155-63.
- Antonsen L, Thayssen P, Maehara A et al. Optical Coherence Tomography Guided Percutaneous Coronary Intervention With Nobori Stent Implantation in Patients With Non-ST-Segment-Elevation Myocardial Infarction (OCTACS) Trial: Difference in Strut Coverage and Dynamic Malapposition Patterns at 6 Months. Circ Cardiovasc Interv 2015;8:e002446.
- Meneveau N, Souteyrand G, Motreff P et al. Optical Coherence Tomography to Optimize Results of Percutaneous Coronary Intervention in Patients with Non-ST-Elevation Acute Coronary Syndrome: Results of the Multicenter, Randomized DOCTORS Study (Does Optical Coherence Tomography Optimize Results of Stenting). Circulation 2016;134:906-17.
- Kala P, Cervinka P, Jakl M et al. OCT guidance during stent implantation in primary PCI: A randomized multicenter study with nine months of optical coherence tomography follow-up. Int J Cardiol 2018;250:98-103.
- Li X, Ge Z, Kan J, Anjum M. lntravascular ultrasound-guided versus angiography-guided percutaneous coronary intervention in acute coronary syndromes (IVUS-ACS): a two-stage, multicentre, randomised trial. Lancet 2024;(Ahead of Print).
Authors





No comments yet!