07 Apr 2024
One-month ticagrelor monotherapy after PCI in acute coronary syndromes: Principal results from the double-blind, placebo-controlled Ultimate DAPT trial
Reported from ACC.24
Elad Asher provides his take on the ULTIMATE-DAPT Trial results which Gregg W Stone presented at ACC.24 in Atlanta.
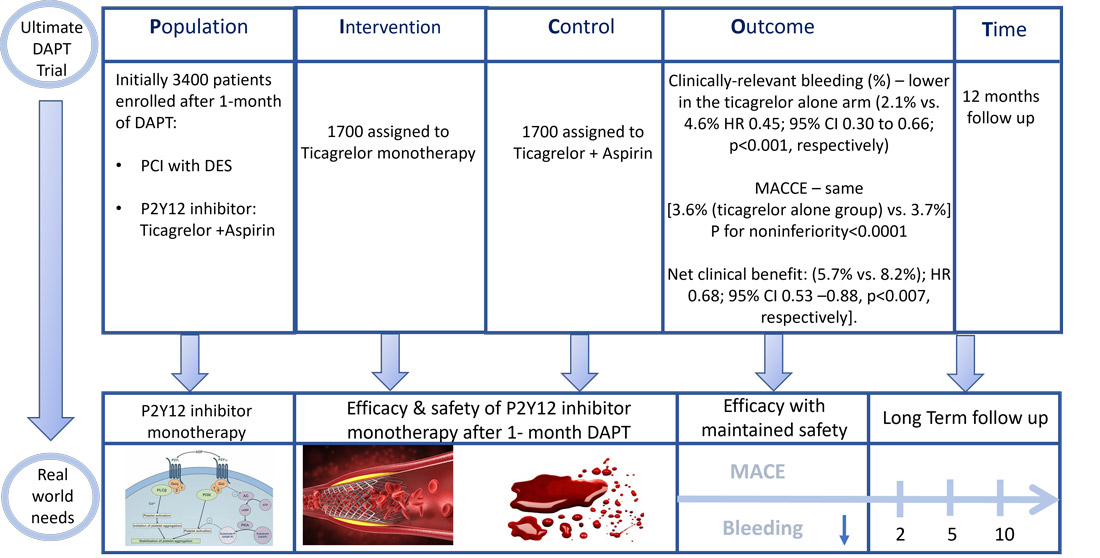
PICOT analysis of Ultimate DAPT Trial - Courtesy of Elad Asher - Source: PCRonline.com
Why this study – the rationale/objective?
- Guidelines currently recommend DAPT with aspirin plus a potent P2Y12 receptor inhibitor for 12 months in most patients presenting with an ACS to prevent MI and stent thrombosis.
- Data regarding the use of single antiplatelet therapy with a potent P2Y12 inhibitor starting 1 month after PCI in ACS is limited.
- The aim of the current trial was to determine whether the use of ticagrelor alone, compared with ticagrelor plus aspirin, after 1-month DAPT could reduce bleeding without increase in major adverse cardiovascular or cerebrovascular events (MACCE) in patients with ACS after PCI.
How was it executed – the methodology?
A large-scale, international, multicenter, placebo-controlled, double-blind randomized trial.
Inclusion Criteria: Patients presenting with ACS within 30 days before randomization:
- ≥18 years of age with either: STEMI/NSTEMI or unstable angina with 1) DS ≥90%, 2) Ruptured plaque, or 3) Thrombotic lesion.
- Patients remained event-free after PCI with DES for one month on ticagrelor plus aspirin.
Exclusion criteria:
- Stroke within 3 months or any permanent neurologic deficit; Previous CABG; Any planned surgery within 12 months; eGFR <20 ml/min/1.73 m²; Need for chronic oral anticoagulation; Life expectancy <1 year; Any condition likely to interfere with study processes.
Primary Outcomes:
Assessed between 1 and 12 months post-PCI
- Effectiveness: Clinically relevant bleeding (BARC types 2, 3, or 5), powered for superiority testing.
- Safety: Composite MACCE, including cardiac death, MI, ischemic stroke, definite stent thrombosis, or clinically driven TVR, powered for non-inferiority testing.
3505 patients with ACS who underwent PCI were enrolled at 58 centres in China (N=52), Pakistan (N=4), the UK (N=1), and Italy (N=1) between August 20, 2019 and October 27, 2022. Of them, 105 patients were not randomized and hence 3400 patients underwent a 2nd randomization at 30 days.
3400 patients were randomized 1:1 (stratified by ACS type, diabetes, IVUS vs angio guidance, and site) and F/U for 1 year.
Major findings
- Clinically-relevant bleeding (%) – lower in the ticagrelor alone arm (2.1% vs. 4.6% HR 0.45; 95% CI 0.30 to 0.66; p<0.001, respectively)
- Clinically-relevant bleeding sub-group analysis – No significant interactions were present in 12 pre-specified subgroups
- Primary Safety Endpoint: MACCE – same between both groups [3.6% (ticagrelor alone group) vs. 3.7%]; Absolute difference -0.1%; 95% CI -1.4% to 1.2%; HR 0.98; 95% CI 0.69 to 1.39. P for noninferiority<0.0001, P for superiority=0.89
- MACCE sub-group analysis – No significant interactions were present in 12 pre-specified subgroups, except possibly for age
- Net adverse clinical events (NACE): MACCE or BARC types 1-5 bleeding, lower in the ticagrelor alone arm [97 (5.7%) vs. 140 (8.2%); HR 0.68; 95% CI 0.53 –0.88, p<0.007, respectively].
Critical reading and the relevance for clinical practice
The authors for the current trial concluded that in patients with ACS treated with PCI who are free from MACCE events after 1 month on DAPT, treatment with ticagrelor alone between 1 and 12 months will decrease clinically relevant and major bleeding while providing similar protection from MACCE compared with ticagrelor plus aspirin.
Furthermore, they think that these results, following prior trials, warrant updating the guidelines and change in practice to treat most patients with ACS after PCI and 1-month DAPT, only followed by conversion to single antiplatelet therapy with a potent P2Y12 inhibitor (with the strongest evidence to date supporting ticagrelor).
Should common practice and guidelines be changed?
The Ultimate-DAPT trial has several limitations: 1) The primary efficacy endpoint included minor bleeding (BARC type 2), however, major bleeding was also significantly reduced with ticagrelor monotherapy (BARC types 3 or 5, TIMI major or minor, GUSTO and ISTH); 2) ~40% of patients had biomarker-negative unstable angina. Nevertheless, the authors think that due to the fact that hs-troponin assays were not widely available in China and Pakistan during the enrollment period, it is likely that many of these patients had NSTEMI; 3) 88.1% of patients were from China, possibly affecting the generalizability of the results.
What would be the future of P2Y12 inhibitor monotherapy in patients with ACS?
Do you think we have enough data to support changing the guidelines as suggested by the authors?




1 comment
Would be too soon, upon available results to recommend to all ACS P2Y1 monoTx after one month DAPT. But, we could leave to to personal, individualize approach, just the freedom in HBR, fragile pts, lots of comorb. older ones.. As a procen posibillity. Because, now there are inter doctoral missunderstanding concerning DAPT & SAPT