Left Atrial Appendage Closure after Ablation for Atrial Fibrillation - the OPTION Trial
Reported from AHA 2024
Alessandro Sticchi provides his take on the OPTION Trial presented at AHA 2024 in Chicago.

Why This Study – The Rationale/Objective
The OPTION trial was conducted to address an important question in the management of patients with atrial fibrillation (AF) who undergo catheter ablation. While current guidelines recommend continued oral anticoagulation (OAC) to prevent stroke in these patients, OAC entails significant limitations, including bleeding risks and adherence challenges. Left atrial appendage closure (LAAC) presents a possible mechanical alternative for stroke prevention that could reduce long-term reliance on OAC. The main objective of the OPTION trial was to determine whether LAAC using the WATCHMAN FLX device (Boston Scientific) could offer noninferior efficacy in preventing death, stroke, or systemic embolism while being safer in reducing non–procedure-related bleeding, compared to OAC over a 36-month period.
How Was It Executed – The Methodology
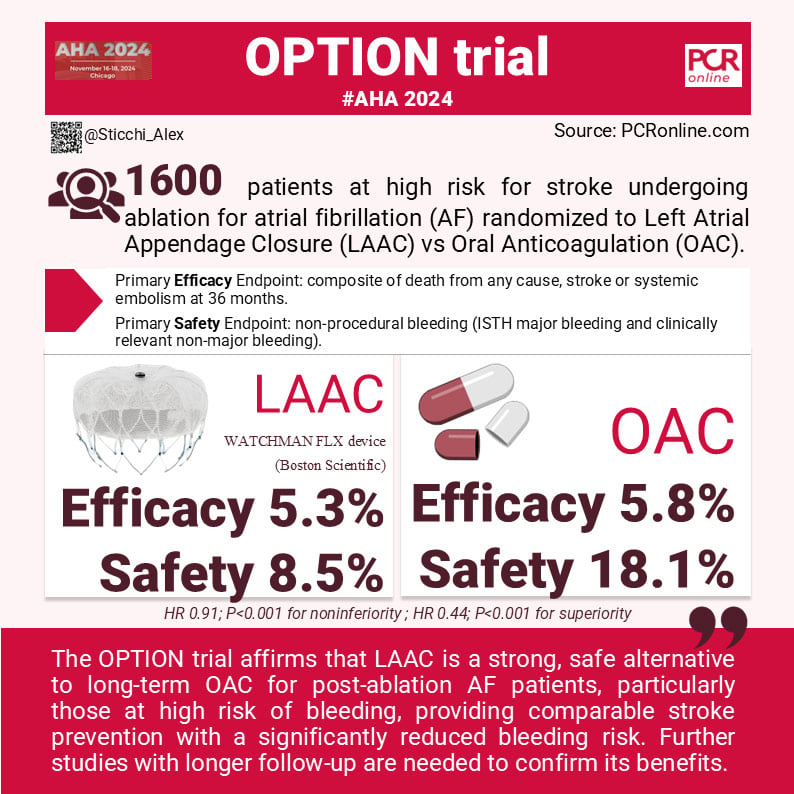
The OPTION trial (ClinicalTrials.gov number, NCT03795298) was a multicenter, randomized, controlled trial conducted at 114 sites in 10 countries (North America, Europe and Oceania). It enrolled 1600 patients who met specific criteria:
Inclusion Criteria:
- Non-valvular AF with catheter ablation performed 90 to 180 days before randomization or planned within 10 days post-randomization.
- CHA2DS2-VASc score of ≥2 for men and ≥3 for women, indicating a moderate to high risk of stroke.
Exclusion Criteria:
- Severe left ventricular dysfunction (ejection fraction ≤30%).
- Stroke or transient ischemic attack within 60 days prior to randomization.
- Recent major bleeding (within 14 days).
- Conditions necessitating long-term anticoagulation unrelated to AF.
- any cardiac or major non-cardiac interventional or surgical procedure (excluding non-valvular AF ablation and cardioversion) within 30 days prior to or 60 days after randomization.
Study Protocol:
Patients were randomly assigned in a 1:1 ratio to either the LAAC group, receiving the WATCHMAN FLX device, or the OAC group, continuing OAC therapy. In the LAAC arm, patients initially received anticoagulants and aspirin for 90 days post-procedure, followed by aspirin monotherapy until 12 months. Follow-up imaging, using TEE or CT, was conducted at 3 and 12 months to check for peridevice leaks or device-related thrombosis. Regular clinical follow-ups occurred at 3, 12, 24, and 36 months.
Endpoints:
- Primary Efficacy Endpoint: LAAC is non-inferior for the composite of death from any cause, stroke (ischemic and/or hemorrhagic), or systemic embolism at 36 months.
- Primary Safety Endpoint: LAAC is superior for non-procedural bleeding (ISTH major bleeding and clinically relevant non-major bleeding).
- Secondary Endpoint: LAAC is non-inferior for ISTH major bleeding (including procedural bleeding).
What are the Main Results?
The OPTION trial met all its endpoints. The trial found that LAAC significantly reduced non–procedure-related bleeding compared to OAC, specifically: 59.3% apixaban, 27.2% rivaroxaban, 4.3% edoxaban, 3.9% dabigatran, 0.3% other and 5% warfarin).
The primary efficacy endpoint showed noninferiority of LAAC compared to OAC, with an event rate of 5.3% in the LAAC group and 5.8% in the OAC group (hazard ratio, 0.91; P<0.001 for noninferiority). The ischemic event rate in both groups was lower than expected, given the mean CHA2DS2-VASc score of 3.5. This reflects effective stroke prevention by both strategies, though the margin for noninferiority was exactly at 5%, suggesting a fine balance between the two approaches.
The incidence of non–procedure-related major or clinically relevant nonmajor bleeding was 8.5% in the LAAC group, compared to 18.1% in the OAC group (hazard ratio, 0.44; P<0.001 for superiority). This marked reduction was largely attributed to a decrease in gastrointestinal bleeding and epistaxis.
The secondary endpoint analysis, covering major bleeding including procedural events, showed 3.9% in the LAAC group versus 5.0% in the OAC group, meeting the criterion for noninferiority (P<0.001), but not achieving superiority (P=0.28).
Critical Reading and the Relevance for Clinical Practice
The OPTION trial provides robust evidence that left atrial appendage closure (LAAC) is a viable alternative to long-term oral anticoagulation (OAC) for patients with atrial fibrillation (AF) who have undergone catheter ablation. The trial's results demonstrated a significant reduction in non–procedure-related bleeding for LAAC compared to OAC, highlighting a marked reduction in events such as gastrointestinal bleeding and epistaxis. This reduction underscores LAAC’s ability to address one of OAC’s critical limitations—its association with both major and non-major bleeding complications.
These findings align with previous landmark studies, including PROTECT AF, PREVAIL, and PRAGUE-17. Making some consideration on the OPTION trial's mean CHA2DS2-VASc score, it was 3.5 (3.5 LAAC vs 3.5 NOAC) indicates a moderate-to-high risk population, similar to PROTECT AF (3.7 LAAC vs 3.4 VKA) and PREVAIL (3.9 VKA vs 3.8 LAAC), but lower than PRAGUE-17 (4.7 LAAC vs 4.7 NOAC). This suggests that OPTION evaluated a cohort mostly similar to the previous studies, reaffirming LAAC's benefits in these patients. About sex, OPTION female representation was around 30%, similar to PRAGUE-17’s (33%) , suggesting that future studies should include more balanced gender representation given women’s higher stroke risk in AF.
In terms of efficacy, the OPTION trial demonstrated noninferiority of LAAC to OAC for preventing death, stroke, and systemic embolism, aligning with outcomes from earlier studies. PROTECT AF reported a 3.0% annualized event rate for LAAC versus 4.3% for warfarin, while PRAGUE-17 showed comparable stroke prevention between LAAC and NOAC (20.0 for LAAC% vs 24.9% for NOAC at 3 years). Safety profiles across these studies consistently supported LAAC's advantage in reducing major bleeding: OPTION reported an 8.5% nonprocedural bleeding rate for LAAC versus 18.1% for OAC, differing from PRAGUE-17's finding of a 4.3% annual major bleeding rate for LAAC compared to 5.8% for NOAC (HR 0.75 [0.44-1.27]).
Although the OPTION trial showed promising three-year data, AF remains a chronic condition with potential asymptomatic recurrences, necessitating ongoing monitoring and further long-term studies to confirm the sustained safety and efficacy of LAAC. The approximately 2.8% of patients lost to follow-up could influence the interpretation of long-term outcomes, a challenge also observed in earlier trials.
In conclusion, OPTION reinforces that LAAC is an effective addition to stroke prevention strategies for post-ablation AF patients, particularly those at elevated bleeding risk. It supports personalized treatment approaches, balancing efficacy and safety, and highlights the importance of continuous follow-up and comprehensive patient risk assessment to optimize outcomes.
References:
Authors: Oussama M. Wazni et al. for the OPTION Trial Investigators.
Published November 16, 2024, DOI: 10.1056/NEJMoa2408308




No comments yet!