TRI.fr trial - Multicentric randomised evaluation of the transcatheter edge-to-edge repair in the treatment of severe isolated secondary tricuspid regurgitation
Reported from ESC Congress 2024
Alex Sticchi provides his take on the results of the TRI.FR trial presented by Erwan Donal at the ESC Congress 2024 in London.
Why this study? The rationale/objective
Severe symptomatic secondary tricuspid regurgitation (TR) presents a significant treatment challenge in high-risk patients. Previous studies have highlighted the potential of Transcatheter Edge-to-Edge Repair (TEER) in reducing TR severity and improving quality of life. However, the impact on hard clinical outcomes, such as mortality and hospitalization, remains underexplored. The TRI.Fr trial aimed to assess whether adding TEER to optimal medical therapy could enhance clinical outcomes more effectively than medical therapy alone in this complex patient population.
How was it executed? The methodology
The TRI.fr trial (ClinicalTrials.gov Identifier: NCT04646811) is a multicenter, superior, open-label, parallel-group, randomized controlled trial designed to evaluate the efficacy and safety of Transcatheter Edge-to-Edge Repair (TEER) combined with Guideline-Directed Medical Therapy (GDMT) versus GDMT alone in patients with severe symptomatic secondary tricuspid regurgitation (TR). The trial was conducted across 24 centers in France and Belgium, enrolling 300 patients who were considered ineligible for corrective action on the valve by a surgical approach after Heart Team discussion.
Study design and procedures:
Patients were randomized in a 1:1 ratio to receive either TEER plus GDMT or GDMT alone. TEER procedures were performed using TriClip G4 (Abbott, Santa Clara, CA, USA). The study design included the primary endpoint through a Packer clinical composite score, which is a three-level ordered categorical endpoint, with each randomized patient being classified as improved, unchanged, or worse due to the clinical response over the follow-up period and at its endpoint at 12 months.
Endpoints:
Primary endpoint:
- Clinical composite score: The primary endpoint was defined as an improvement in the hierarchical clinical composite score over 12 months. This score included:
- Incidence of major adverse cardiovascular events (MACE), including cardiovascular death and hospitalization.
- Improvement in New York Heart Association (NYHA) functional class.
- Patient Global Assessment (PGA) of health and symptom improvement.
Secondary endpoints:
- Reduction in TR severity: Assessed through echocardiographic evaluation, aiming for a reduction in TR severity to less than severe at 12 months.
- Quality of life improvements: Measured by changes in KCCQ scores from baseline to 12 months.
- NYHA functional class improvement: The proportion of patients showing at least a one-class improvement in NYHA functional class.
- Heart failure hospitalizations: The rate of hospitalizations due to heart failure over the 12-month follow-up period.
- All-cause mortality: The rate of all-cause mortality at 12 months.
What are the main results?
The TRI.fr trial revealed detailed findings in terms of clinical outcomes, device performance, and patient quality of life improvements.
- Primary endpoint:
- Clinical composite score: 74.1% of patients in the TEER group showed improvement compared to 40.6% in the GDMT group. The effect estimate was 0.67 (95% CI: 0.61-0.72; P < .0001), indicating a significant improvement with TEER.
- Secondary endpoints:
- TR grade Reduction: At 12 months, 93.2% of patients in the TEER group achieved a TR grade reduction to less than 4+, compared to only 46.5% in the GDMT group. The effect estimate for this was 0.73 (95% CI: 0.68-0.78; P < 0.0001).
- KCCQ overall summary score: There was a significant increase in the KCCQ score from baseline to 12 months in the TEER group (69.9 ± 25.5) compared to the GDMT group (55.4 ± 28.8), with an absolute difference of 14.5 points (P < 0.001).
- Patient global assessment: Improvement was noted in 74.6% of the TEER group versus 39.5% of the GDMT group. The effect estimate was 0.68 (95% CI: 0.63-0.74; P < 0.0001).
- Device performance:
- Technical success: Achieved in 97.3% of the procedures.
- Number of devices implanted: The majority of patients (68.2%) received 2 devices, indicating the need for multiple devices to achieve optimal results.
- Device type used: 98.3% of the devices used were XT or XTW, highlighting a preference or better suitability of these types for the procedures involved.
- Safety and complications:
- Major adverse events: There was a low incidence of in-hospital complications (8%) and single leaflet device attachment (5.2%). There were no per-procedural deaths, and in-hospital mortality was 0.6%.
- Hierarchical clinical composite endpoint:
- This metric combined time to death, tricuspid valve surgery, heart failure hospitalizations, and improvement of ≥ 15 points in KCCQ score at 1 year. The effect estimate was 2.06 (1.38-3.08; P = .0004), demonstrating substantial benefits from TEER.
- Kaplan-Meier estimates:
- Free from MACE through 1 year: There was no statistically significant difference between groups, with the TEER group at 0.78 and the GDMT at 0.84 (P = 0.38).
- Free from cardiovascular death at 1 year: Slightly higher survival in the TEER group compared to GDMT (0.966 vs. 0.942), although the difference was not statistically significant (P = 0.37).
Critical reading and relevance for clinical practice
The TRI.fr trial stands as a pivotal study in the ongoing evolution of transcatheter interventions for tricuspid regurgitation (TR), particularly for patients at high surgical risk. By demonstrating not only the procedural success of Transcatheter Edge-to-Edge Repair (TEER) but also its sustained impact on long-term clinical outcomes and quality of life, the TRI.fr trial is instrumental in validating TEER as a viable treatment alternative for severe TR. The trial’s findings strongly suggest that TEER should be more widely considered in clinical practice for its safety and strong impact on quality of life.
Comparative analysis with TRILUMINATE
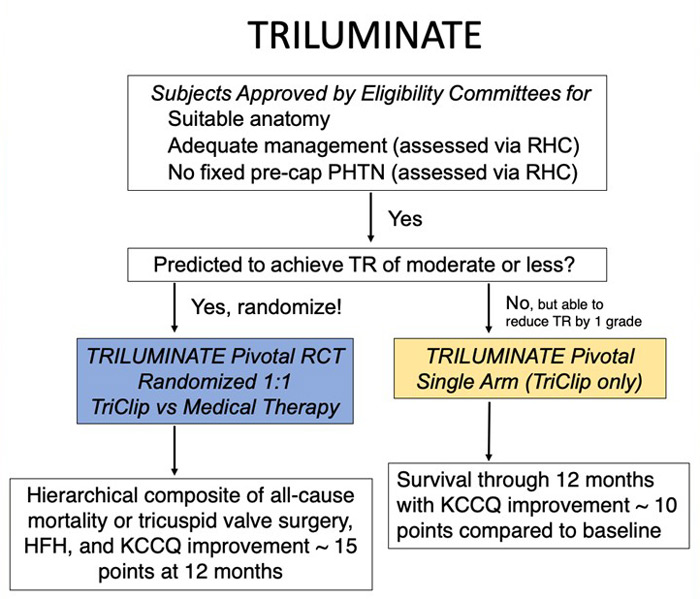
In comparing the TRI.fr and TRILUMINATE trials, we can start from the study design, and we can see in the following dedicated flowcharts that there are no significant differences in the rigorous selection process through the eligibility committee. The only difference we can highlight is the follow-up longer than 1 year and no crossover of the TRI.fr compared to the TRILUMINATE.
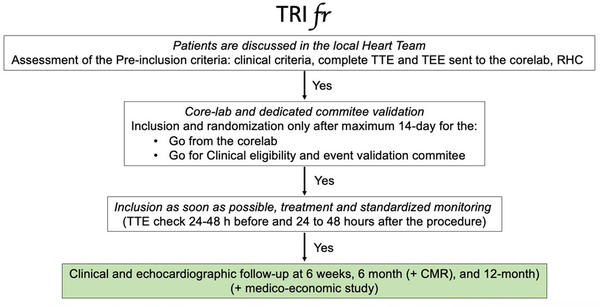
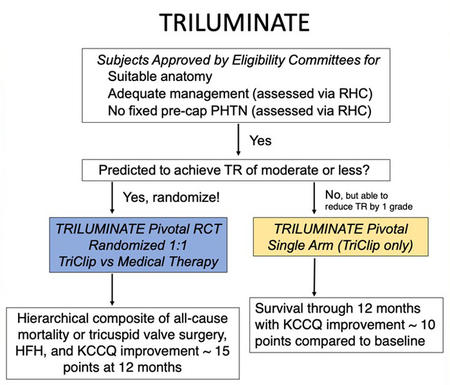
The similarity also extends to the key inclusion and exclusion criteria, which could be considered an endorsement of TRILUMINATE’s patient selection process. Both trials specifically targeted patients with symptomatic secondary tricuspid regurgitation (TR), placing a strong emphasis on anatomical suitability for Transcatheter Edge-to-Edge Repair (TEER). This focus is particularly important given the growing number of devices available for both orthotopic and heterotopic tricuspid valve replacement, with emerging favourable evidence from early studies.
Criteria \ Trial | TRILUMINATE | TRIFR |
|---|---|---|
Key Inclusion Criteria | Patients with severe TR who remain symptomatic despite medical therapy. Tricuspid regurgitation confirmed by an independent echocardiography laboratory as severe despite medical therapy. | Symptomatic ≥ severe TR , but stable for ≥ 30 days.
|
Key Exclusion Criteria | Indication for other valve disease intervention. Indication for left-sided (e.g. severe aortic stenosis, severe mitral regurgitation) or pulmonary valve correction prior 60 days). | M-TEER, CRT, Myocardial infarction or coronary bypass surgery in the past 3 months. |
These trials had a dedicated committee to assess anatomical eligibility for tricuspid TEER, highlighting the importance of anatomical criteria selection for procedural success and, consequently, favourable outcomes.
Efficacy in reducing TR severity: The TRI.fr trial reported that 71.0% of patients achieved a reduction in TR severity to less than severe at 12 months post-intervention. This aligns with the results of the TRILUMINATE trial, where 89% of patients reached moderate or less TR at one year. The slight differences in these outcomes likely reflect variations in patient populations, with TRI.fr having 99% of patients presenting with massive and torrential TR at baseline, compared to 72% in the TRILUMINATE trial. Nevertheless, both trials clearly demonstrate the efficacy of TEER in significantly reducing TR severity.
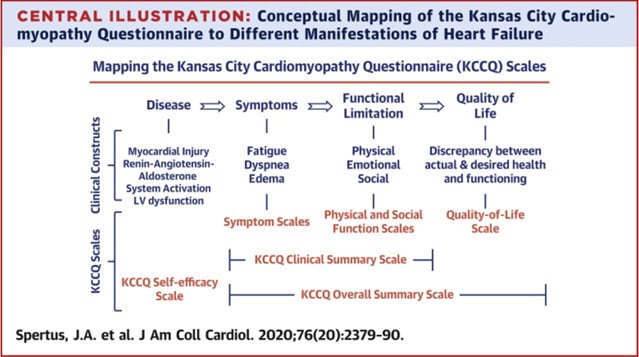
Improvements in quality of life: Quality of life improvements were a key outcome in both trials. In TRI.fr, patients experienced an absolute difference of 14.5 points in the Overall Kansas City Cardiomyopathy Questionnaire (KCCQ) scores at 12 months between the interventional and control groups (p<0.001). The Overall KCCQ is a more comprehensive tool for life assessment than the version used in TRILUMINATE, as illustrated in the elegant figure by Spertus et al. However, the TRILUMINATE trial reported a KCCQ score mean difference (T-TEER vs control group) of 18 points at the 1-year follow-up (p<0.0001), confirming the significant symptomatic relief and enhancement in daily living that TEER provides to patients suffering from TR.
Safety and procedural success: Both TRI.fr and TRILUMINATE trials reported high procedural success rates, with TRI.fr achieving a 97.3% success rate and TRILUMINATE an impressive 98.8%. The safety profiles were similarly robust, with TRI.fr reporting a low rate of major adverse events and TRILUMINATE indicating that 98.3% of patients were free from major adverse events at 30 days. These results affirm that TEER is not only effective but also safe, particularly in a population that often presents with significant comorbidities.
Long-term outcomes and sustained efficacy: The long-term benefits observed in the TRI.fr trial, including sustained TR reduction and quality of life improvements, align closely with the findings from TRILUMINATE. Although TRILUMINATE did not show a significant difference in hospitalization rates for heart failure between the groups, the overall improvements in TR severity and patient-reported outcomes suggest that TEER can play a crucial role in managing severe TR over the long term.
Unfortunately, due to study design, patients in TRILUMINATE can perform a crossover after the one-year follow-up and this makes to miss the opportunity to reach a possible significant difference for hard outcomes such as mortality. The TRI.fr will give us this chance to have a follow-up longer than one year and see if we will reach this goal in the tricuspid repair scenario.
Implications for clinical practice:
The results from the TRI.fr and TRILUMINATE trials collectively reinforce the potential of TEER to become a cornerstone treatment for severe tricuspid regurgitation. These trials highlight the need for broader adoption of TEER in clinical practice, supported by advancements in patient screening and procedural technologies that enhance outcomes. Looking to the future, continued research and development in this field are likely to further refine TEER techniques, improve patient selection, and potentially expand the indications for this life-improving intervention.
The future of TEER in managing tricuspid regurgitation (TR) appears promising, with the potential to broaden its indications beyond high-risk patients to become a first-line treatment option. Technological innovations, particularly in imaging and device development, are expected to enhance procedural success and safety, making TEER more accessible. As TEER becomes integral to heart failure management, with its proven ability to reduce hospitalizations and improve quality of life, personalized treatment approaches will likely emerge, optimizing outcomes based on individual patient characteristics. As long-term data accumulates, TEER is poised to gain global acceptance, potentially reshaping guidelines and becoming a standard treatment for severe TR.
Related publications
- Transcatheter treatment for tricuspid valve disease
- Transcatheter edge-to-edge valve repair versus minimally invasive beating-heart surgery of the tricuspid valve: an observational study
- One-year outcomes of transcatheter tricuspid valve repair with the Mistral device




No comments yet!