Large-bore mechanical thrombectomy vs catheter-directed thrombolysis for treatment of intermediate-risk pulmonary embolism: primary outcomes from the PEERLESS randomized controlled trial
Reported from TCT 2024
Elad Asher provides his take on the PEERLESS trial presented by Wissam A. Jaber at TCT 2024 in Washington.
Why this study – the rationale/objective?
- Although large-bore mechanical thrombectomy (LBMT) and catheter-directed thrombolysis (CDT) have been reported with positive outcomes in patients with intermediate-risk pulmonary embolism (PE), there are no randomized controlled trials comparing these two techniques.
- The aim of the current trial was to compare these two advanced therapies in the management of acute intermediate-risk PE.
How was it executed – the methodology?
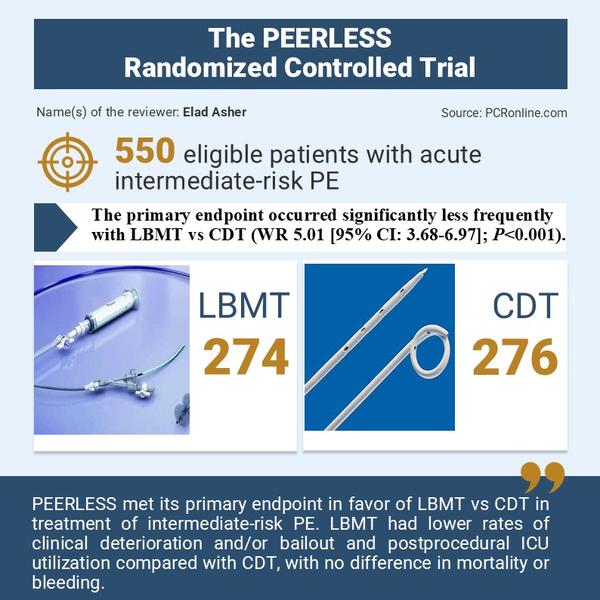
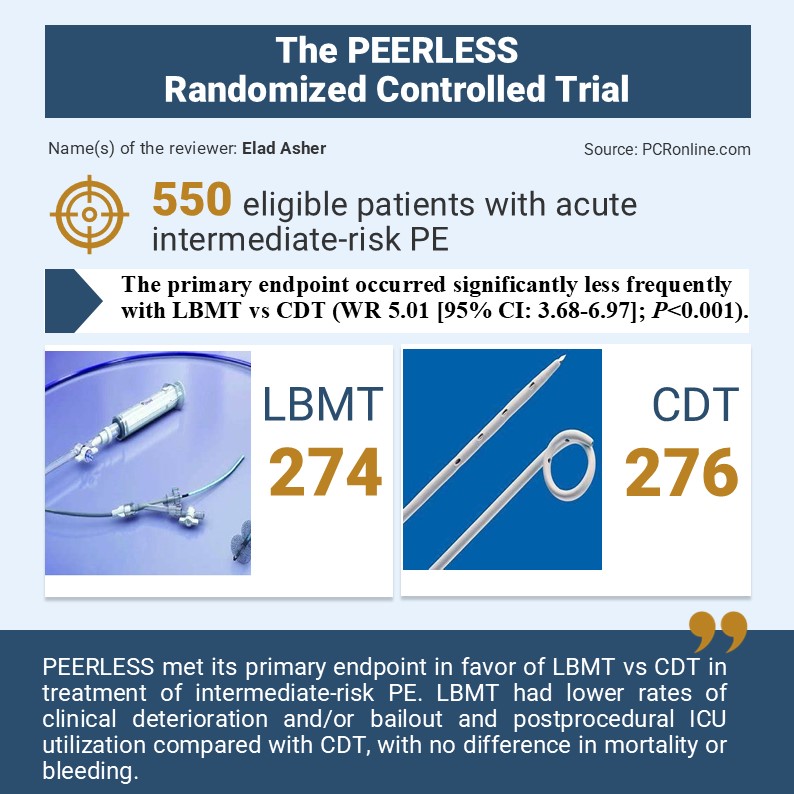
A prospective, multicentre, randomized control trial which enrolled 550 intermediate-risk PE patients with right ventricular dilatation and additional clinical risk factors randomized 1:1 to treatment LBMT or CDT.
- Inclusion criteria: ≥18 years of age with an intermediate-risk PE. In addition to RV dilatation or dysfunction on computed tomographic pulmonary angiograms (CTPA) or echocardiogram.
Patients were required to have a proximal filling defect in ≥1 main or lobar pulmonary artery, symptom duration ≤14 days, and intervention planned ≤72 hours from diagnosis or arrival from a transferring hospital.
(The original study protocol required patients to have elevated cardiac troponin levels. The protocol was amended to include elevated troponin in a broader list of other clinical risk factors [history of heart failure, history of chronic lung disease, heart rate ≥110 bpm, systolic blood pressure <100 mmHg, respiratory rate ≥30 rpm, oxygen saturation <90%, syncope related to PE, elevated lactate ≥1]).
- Exclusion criteria: Patients who could not receive therapeutic anticoagulation, right heart clot-in transit, life expectancy was <30 days, systolic pulmonary artery pressure (sPAP) was ≥70 mmHg on invasive hemodynamic measurement at the start of the index procedure prior to insertion of the therapeutic catheter.
- Primary endpoint: a hierarchal win ratio (WR) composite of the following: 1) all-cause mortality, 2) intracranial haemorrhage, 3) major bleeding, 4) clinical deterioration and/or escalation to bailout, and 5) postprocedural intensive care unit (ICU) admission and length of stay (the sooner of hospital discharge or 7 days post-procedure).
- Assessments at the 24-hour visit included respiratory rate, dyspnea score, NYHA classification, right ventricle (RV)/left ventricle (LV) ratio reduction, and RV function.
- Endpoints through 30 days included total hospital stay, all-cause readmission, and all-cause mortality.
Results
Major findings:
- The primary endpoint occurred significantly less frequently with LBMT vs CDT (WR 5.01 [95% CI: 3.68-6.97]; P<0.001).
- There were significantly fewer episodes of clinical deterioration and/or bailout (1.8% vs 5.4%; P=0.04) with LBMT vs CDT
- Less postprocedural ICU utilization (P<0.001), including admissions (41.6% vs 98.6%) and stays >24 hours (19.3% vs 64.5%)
- There was no significant difference in mortality, intracranial hemorrhage, or major bleeding between strategies, nor in a secondary WR endpoint including the first 4 components (WR 1.34 [95% CI: 0.78-2.35]; P=0.30).
- Less RV dysfunction (42.1% vs 57.9%; P=0.004), nevertheless, RV/LV ratio reduction was similar (0.32±0.24 vs 0.30±0.26; P=0.55).
- LBMT patients had shorter total hospital stays (4.5±2.8 vs 5.3±3.9 overnights; P=0.002) and fewer all-cause readmissions (3.2% vs 7.9%; P=0.03)
- 30-day mortality was similar (0.4% vs 0.8%; P=0.62)
- Safety was similar with either intervention as well, with no difference between the LBMT procedure and CDT in serious adverse events related to the device or drug through 30 days (13.3% vs 11.5%; P = 0.59)
Critical reading and the relevance for clinical practice
The authors of the current trial concluded that PEERLESS met its primary endpoint in favour of LBMT vs CDT in the treatment of intermediate-risk PE. LBMT had lower rates of clinical deterioration and/or bailout and postprocedural ICU utilization compared with CDT, with no difference in mortality or bleeding.
Nevertheless, this finding favouring LBMT arises mainly from the two components: (i) significantly lower rates of clinical deterioration and/or escalation to bailout therapy and (ii) significantly less frequent ICU admissions and shorter postprocedural ICU lengths of stay (with no significant differences in in-hospital mortality or major bleeding between arms).
Should common practice and guidelines be changed?
The PEERLESS trial has several limitations: 1) an open-label trial in which participants and investigators were unblinded to treatment. 2) treatment in the CDT arm was not standardized. 3) The CDT arm included patients treated with ultrasound-facilitated CDT and conventional CDT methods. 4) patient follow-up was limited to a 30-day visit.
What do you think would be the future of catheter-directed treatment in patients with acute intermediate-risk PE? Do you think we have sufficient data to choose catheter treatment over pharmacological treatment only? Do you think LBMT is better than CDT for the treatment of acute intermediate PE?





No comments yet!