TRISCEND II trial: Randomized comparison of transcatheter valve replacement vs. optimal medical therapy for severe tricuspid regurgitation
Reported from TCT 2024
Alex Sticchi provides his take on the results of the TRISCEND II trial presented by Susheel K. Kodali and Suzanne V. Arnold at TCT 2024 in Washington.
Why this study? – The rationale/objective
Severe tricuspid regurgitation (TR) remains a challenging condition with limited effective treatment options. TR is associated with high morbidity and mortality, particularly among elderly patients with co-morbidities such as atrial fibrillation and heart failure. Traditional surgical interventions are often avoided due to high perioperative risks, and while transcatheter edge-to-edge repair (T-TEER) has shown some improvement in symptoms and quality of life, it frequently leaves residual TR, which correlates with worse outcomes.
The TRISCEND II trial aimed to assess whether transcatheter tricuspid valve replacement (TTVR) with the EVOQUE valve system (Edwards Lifesciences) could more effectively reduce TR severity, improve quality of life, and enhance functional capacity compared to medical therapy alone. The primary hypothesis was that TTVR would result in a meaningful improvement in a composite outcome reflecting both clinical and patient-reported endpoints. This trial was designed to address a significant gap in TR management by offering an alternative that could provide effective TR reduction and symptom relief with a minimally invasive approach
How was it executed? – The methodology
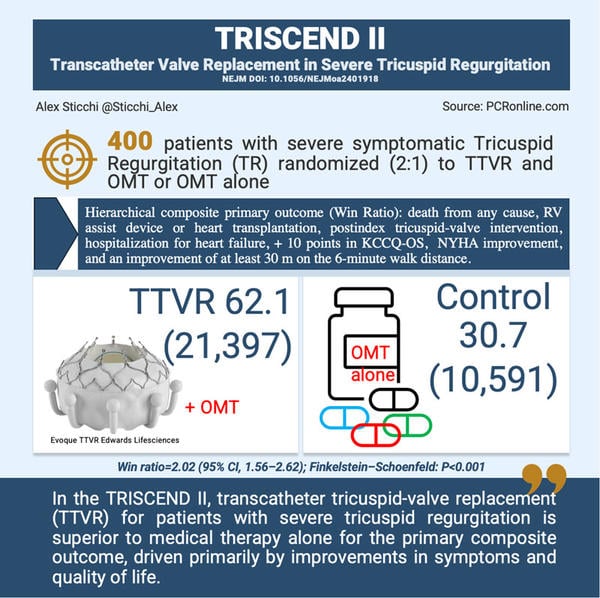
The TRISCEND II trial was a multinational, randomized, controlled study involving 400 patients with symptomatic, at least severe TR. Patients were enrolled across 45 centers in the U.S. and Germany and randomly assigned in a 2:1 ratio to receive either TTVR with the EVOQUE valve plus optimal medical therapy or optimal medical therapy alone.
Key methodology components included:
- Patient Population: Participants were predominantly elderly (mean age 79.2 years), with a high prevalence of comorbidities, including atrial fibrillation (94.9%) and renal insufficiency. Patients with conditions like severely depressed right ventricular function or anatomical incompatibility with the EVOQUE system were excluded.
- Interventions: The TTVR procedure aimed to reduce TR severity to mild or less, monitored via echocardiography. Patients in the TTVR group were advised to receive anticoagulation and aspirin for at least six months post-procedure.
- Primary Outcome: The primary endpoint was a hierarchical composite outcome, including death from any cause, need for right ventricular assist device or heart transplantation, additional tricuspid-valve interventions, hospitalization for heart failure, improvement of ≥10 points on the Kansas City Cardiomyopathy Questionnaire overall summary (KCCQ-OS), improvement in New York Heart Association (NYHA) functional class by at least one level, and a ≥30-meter improvement on the 6-minute walk test.
- Analysis Approach: The hierarchical composite outcome was evaluated using the win-ratio method, allowing for a nuanced comparison across clinical outcomes and patient-reported metrics. The primary population for analysis included all patients who had undergone an attempted procedure or received medical therapy alone.
What is the main result?
The study results demonstrated that TTVR with the EVOQUE system provided significant clinical benefits over medical therapy alone, as shown in both primary and secondary endpoints:
- Primary Composite Outcome: The win ratio of 2.02 (95% CI, 1.56 to 2.62; P<0.001) indicated a clear advantage of TTVR in improving the hierarchical outcome measures.
- Key Components of the Primary Outcome:
- Mortality: At 1-year follow-up, TTVR was associated with a slightly lower mortality rate (12.6%) compared to the control group (15.2%).
- Hospitalization for Heart Failure: TTVR patients experienced a lower hospitalization rate for heart failure (20.9%) compared to controls (26.1%).
- Quality of Life: Improvement in KCCQ-OS scores by at least 10 points was achieved in 66.4% of TTVR patients versus 36.5% in controls, demonstrating a substantial quality-of-life enhancement.
- Functional Improvement (NYHA Class): TTVR patients showed marked improvement in NYHA class, with 78.9% achieving at least one functional class improvement, compared to 24.0% in the control group.
- 6-Minute Walk Distance: Increased physical capacity was observed in 47.6% of TTVR patients (≥30 meters improvement) compared to 31.8% in the control group.
- Secondary Outcomes: TTVR significantly reduced TR severity, with 95.3% of patients achieving mild or less TR at 1 year, compared to only 16.1% of control patients.
- Safety and Adverse Events: TTVR was associated with notable adverse events, including severe bleeding in 15.4% of patients versus 5.3% in the control group (P=0.003), and new pacemaker implantation in 17.4% of TTVR patients compared to 2.3% of controls, highlighting procedural risks.
Critical reading and relevance for clinical practice
The TRISCEND II trial results demonstrate the promise of transcatheter tricuspid valve replacement (TTVR) for patients with severe symptomatic tricuspid regurgitation (TR), yet critical reflections highlight both potential applications and limitations:
- Clinical Impact and Interpretation: The primary benefits of TTVR in this trial—improvements in quality of life, functional status, and TR reduction—are important achievements, especially for a population with few effective alternatives. These improvements reflect a significant step toward managing symptoms that diminish quality of life and physical capacity, aligning with patients’ preferences for symptom relief over purely survival-oriented outcomes.
- Risk-Benefit Balance: Despite the positive findings, we have to highlights notable procedural risks associated with TTVR. The incidence of severe bleeding and new pacemaker implantation—15.4% and 17.4%, respectively—are significant, particularly in a high-risk, elderly cohort. We. need to underscores that these risks necessitate careful patient selection and shared decision-making, ensuring that patients and their families understand both the potential symptomatic benefits and the procedural risks. This discussion is essential to weigh the procedure’s value against the high likelihood of adverse events.
- Comparative Efficacy with T-TEER: TTVR alongside transcatheter tricuspid edge-to-edge repair (T-TEER), a less invasive alternative that, while reducing TR severity less effectively than TTVR, carries a lower risk of periprocedural complications such as bleeding and pacemaker dependency. However, many patients are ineligible for T-TEER due to anatomical constraints, underscoring the need for TTVR in cases where T-TEER is not viable. This comparison underscores TTVR’s unique role in providing meaningful symptom relief, albeit with procedural trade-offs.
- Methodological Considerations and Potential Bias: The trial’s open-label design and the reliance on patient-reported outcomes, both of which introduce potential bias. Patient expectations may influence reported improvements in quality of life, while functional assessments made by clinicians can be imprecise. These factors, along with the relatively short 1-year follow-up and limited sample size, mean that the longer-term benefits and risks of TTVR remain uncertain.
- Future Directions and Clinical Recommendations: We need to continue research to define Tricuspid Intervention and TTVR’s optimal place in clinical practice, particularly as part of a broader TR management strategy. Enhanced recognition and early referral of TR patients could allow intervention before disease progresses to advanced stages. Further, as TTVR technology and procedural expertise evolve, post-approval studies mandated by regulatory bodies will be vital in providing additional safety data and refining patient selection criteria. Improvements in device design, pacemaker lead management, and operator training are key areas that could enhance the procedure’s safety and expand its accessibility across diverse clinical settings.
In summary, the TRISCEND II trial underscores TTVR as a valuable option for symptomatic relief in severe TR patients who are not surgical candidates, marking an advancement in TR treatment. However, careful patient selection, shared decision-making, and ongoing monitoring of long-term outcomes are essential as TTVR finds its place in clinical practice.
References
Transcatheter Valve Replacement in Severe Tricuspid Regurgitation. R.T. Hahn, R. Makkar, V.H. Thourani, M. Makar, R.P. Sharma, C. Haeffele, C.J. Davidson, A. Narang, B. O’Neill, J. Lee, P. Yadav, F. Zahr, S. Chadderdon, M. Eleid, S. Pislaru, R. Smith, M. Szerlip, B. Whisenant, N.K. Sekaran, S. Garcia, T. Stewart‑Dehner, H. Thiele, R. Kipperman, K. Koulogiannis, D.S. Lim, D. Fowler, S. Kapadia, S.C. Harb, P.A. Grayburn, A. Sannino, M.J. Mack, M.B. Leon, P. Lurz, and S.K. Kodali, for the TRISCEND II Trial Investigators. NEJM. DOI: 10.1056/NEJMoa2401918
Watch the interview with Susheel K. Kodali
- Related webinar: TRISCEND II: implications on current clinical practice





No comments yet!