Angiography-derived fractional flow reserve versus intravascular ultrasound to guide percutaneous coronary intervention in patients with coronary artery disease: the FLAVOUR II trial
Reported from ACC.25
Ali Nazmi Calik provides his take on the FLAVOUR II trial presented by Jian-An Wang at ACC.25 in Chicago.
Designed by Ali Nazmi Calik. Source: PCRonline.com
Why this study – The Rationale/Objective
Angiography-derived fractional flow reserve (FFR) and intravascular ultrasound (IVUS) are both used to guide percutaneous coronary intervention (PCI), but their comparative effectiveness, especially in a comprehensive decision-making strategy, had not been well-studied. Angiography-derived FFR, a wire-free physiological assessment, has shown superiority over conventional angiography, but has not proven clear non-inferiority to wire-based FFR in clinical outcomes. IVUS, widely recommended for PCI optimisation, provides detailed anatomical insights for stent decisions, though its comparison with angiography-derived FFR had not been extensively tested in large-scale trials.
The FLAVOUR II trial aimed to address this gap by conducting a head-to-head comparison of PCI strategies guided by angiography-derived FFR and IVUS, in terms of clinical outcomes in patients with angiographically significant stenosis.
How was it executed - The Methodology
The FLAVOUR II trial was a prospective, multicenter, randomised, open-label, non-inferiority trial conducted at 22 centers in China. Patients aged 18 years or older with suspected ischemic heart disease and a de novo stenosis of at least 50 % in a vessel measuring at least 2.5 mm by visual estimation on coronary angiography were eligible for enrollment. Target vessels were limited to major epicardial coronary arteries, including the left anterior descending artery, left circumflex artery, and right coronary artery. Exclusion criteria included target vessel total occlusion, target lesions located in coronary artery bypass grafts or the left main coronary artery, and lesions unsuitable for AngioFFR due to myocardial bridging, severe tortuosity, severe overlap, or poor image quality.
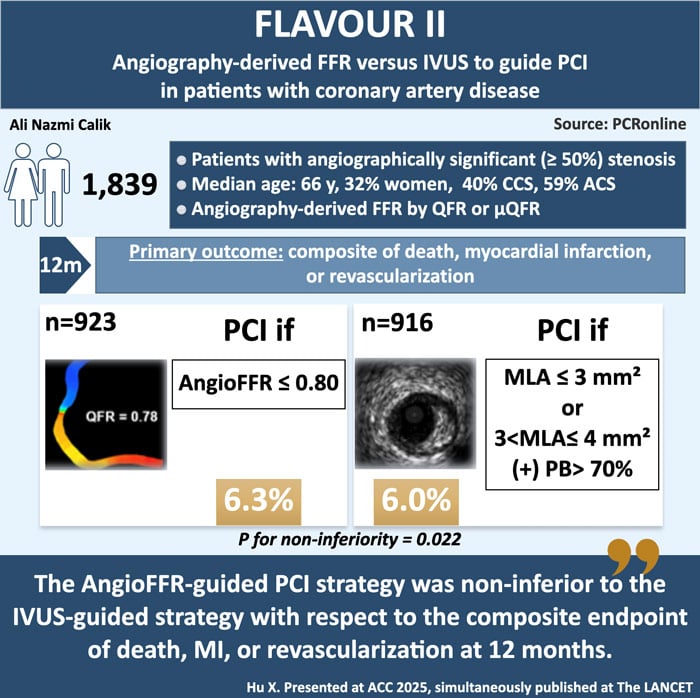
The participants were randomly assigned to either the angiography-derived FFR group or the IVUS group. A total of 1,839 patients were enrolled, with 923 in the FFR group and 916 in the IVUS group. In the angiography-derived FFR group, the criterion for revascularisation was an angiography-derived FFR of 0.80 or less. For the angiography-derived FFR, the quantitative flow ratio (QFR; Shanghai Pulse Medical Technology, Shanghai, China) or Murray law-based QFR (µQFR; Shanghai Pulse Medical Technology) was used1. Optimal PCI was defined as a post-procedural angiography-derived FFR of at least 0.88 or a difference in the angiography-derived FFR across the stent of less than 0.052,3.
In the IVUS group, the criteria for revascularisation were either a minimal lumen area measuring 3 mm² or less or a minimal lumen area measuring 3–4 mm², with a plaque burden of more than 70 %. Optimal PCI was defined as a plaque burden at the stent edge of 55 % or less and a minimal stent area of 5.5 mm² or more or a minimal stent area that was equal to or larger than the distal reference lumen area.
The primary outcome was a composite of death, myocardial infarction, or revascularisation at 12 months post-procedure. The key secondary outcomes included death, MI or revascularisation at 24 and 60 months, target vessel failure (a composite of cardiac death, target-vessel MI, or target lesion revascularisation), all-cause and cardiac death, target-vessel and all-cause nonfatal MI with/without peri-procedural MI, any revascularisation (ischemia-driven or all).
What is the main result?
Between 2020 and 2023, a total of 1,872 patients were successfully enrolled in the trial. After withdrawals, 923 patients were randomly assigned to the angiography-derived FFR group, and 916 to the IVUS group. The median age of the patients was 66 years, with 32.1 % being female. Of 1,839 patients, 736 (40.0 %) had chronic coronary syndrome and 1,087 (59.1 %) had acute coronary syndrome.
The distribution of measured vessel locations, median lesion length, median reference vessel diameter, and median diameter stenosis were similar between the two groups. The median angiography-derived FFR was 0.73 (IQR 0.56–0.84). The median minimal lumen area was 2.68 mm² (IQR 2·19–3·35) and plaque burden was 76.0 % (70.0–81.0), as assessed by IVUS.
The angiography-derived FFR group received fewer stents per patient than the IVUS group (mean number 1.06 [SD 0.90] vs 1.22 [0.92], p < 0.0001). Optimal PCI was achieved in 606 (88.9 %) of 682 patients in the angiography-derived FFR group and 430 (56.5 %) of 761 patients in the IVUS group. The median minimal stent area measured by IVUS was 6·76 mm² (IQR 5.49–8.57).
During the 12-month evaluation period in the time-to-event analysis, the primary outcome event occurred in 56 of 923 patients in the angiography-derived FFR group and 54 of 916 patients in the IVUS group (6.3 % vs 6.0 %, absolute difference 0.2 percentage points [95 % CI –2.0 to 2.4], Pnon-inferiority = 0.002). The pre-specified subgroup analysis showed similar results between the two groups. In a post-hoc per-vessel analysis, the cumulative incidence of target vessel failure was similar between the angiography-derived FFR and IVUS groups.
Critical reading and relevance for clinical practice
The FLAVOUR II trial demonstrated that the angiography-derived FFR-guided PCI strategy was non-inferior to the IVUS-guided PCI strategy in terms of a composite outcome of death, myocardial infarction, or revascularisation at 12 months in patients with angiographically significant coronary stenosis. Moreover, angiography-derived FFR guidance led to fewer PCI procedures compared to IVUS guidance, resulting in reduced stent implantations and less frequent use of dual antiplatelet therapy.
Previous studies have shown that clinical outcomes are better when both physiological assessment and intravascular imaging are used, compared to angiography alone. Physiological assessment primarily improves cardiovascular outcomes by identifying coronary stenoses that are prognostically significant and likely to benefit from revascularisation. In contrast, intravascular imaging enhances outcomes by optimising the PCI procedure, including selecting the appropriate stent size, ensuring precise stent placement, and addressing suboptimal stent implantation4-8.
Despite the common complementary use of physiological and anatomical assessments in daily practice, the results of the FLAVOUR II study suggests that, when physicians prefer a single adjunctive approach to conventional coronary angiography for managing non-complex coronary lesions, angiography-derived FFR is an effective option for both lesion selection and stent optimisation, especially when the aim is to avoid unnecessary PCI. Conversely, if a more proactive PCI strategy is desired to potentially reduce the risk of future coronary events, IVUS can be used for treatment planning and procedural optimisation.
In the FLAVOUR II trial, the proportion of patients with optimal PCI was higher in the angiography-derived FFR group than in the IVUS group (88.9 % vs. 56.5 %). This 56.5 % rate is consistent with previous IVUS studies, including IVUS-XPL (54 %) (5), FLAVOUR (54.8 %) (9), and ULTIMATE (53 %)10. With the PCI optimisation criteria used in this trial, similar PCI results were seen between the two groups, characterised by similar residual stenosis and post-PCI angiography-derived FFR. Moreover, the angiography-derived FFR guidance led to similar composite outcomes to the IVUS guidance, and the results remained consistent even in the patients who underwent PCI. The previous IVUS trials have shown that IVUS-guided PCI leads to larger and longer stents compared to angiography-guided PCI7,11. Interestingly, in the current study, the stent sizes were similar between the two groups, which may be due to the quantitative anatomical information provided by angiography-derived FFR.
An important strength of the trial is the use of next-generation µQFR technology, which addresses the limitations of conventional angiography-derived FFR, such as the need for two angiographic projections and operator variability. This AI-enhanced algorithm enables faster, more automated, and reproducible FFR computation from a single-view angiogram1.
One should bear in mind the limitations of the study when implementing the results of the trial into daily practice. The study population had relatively low anatomical complexity, with a median SYNTAX score of 9. Second, the PCI criteria in the IVUS group may have contributed to the differing PCI rates, as no definitive standard exists for decision-making regarding revascularisation with IVUS. An absolute criterion of minimal lumen area of 3 mm² or less using IVUS led to a higher revascularisation rate in the distal portion of the vessel compared to angiography-derived FFR. Third, although the findings met the prespecified non-inferiority margin, the observed event rates were lower than expected. This could limit the generalisability of the results to high-risk populations, where the event rate difference might exceed the predefined non-inferiority margin. Fourth, target lesion selection was based on the physician’s clinical judgment, which limits the assessment of angiography-derived FFR’s feasibility for evaluating multiple lesions simultaneously.
To conclude, in patients with non-complex coronary artery disease, the angiography-derived FFR-guided comprehensive PCI strategy, which includes both decision-making and stent optimisation, demonstrated comparable effectiveness to the IVUS-guided strategy in terms of the composite endpoint of death, myocardial infarction, or revascularisation at 12 months.
References
- Tu S, Ding D, Chang Y, Li C, Wijns W, Xu B. Diagnostic accuracy of quantitative flow ratio for assessment of coronary stenosis significance from a single angiographic view: a novel method based on bifurcation fractal law. Catheter Cardiovasc Interv 2021; 97 (suppl 2): 1040–47.
- Uretsky BF, Agarwal SK, Vallurupalli S, et al. Trans-stent FFR gradient as a modifiable integrant in predicting long-term target vessel failure. JACC Cardiovasc Interv 2022; 15: 2192–202.
- Li S-J, Ge Z, Kan J, et al. Cutoff value and long-term prediction of clinical events by FFR measured immediately after implantation of a drug-eluting stent in patients with coronary artery disease: 1- to 3-year results from the DKCRUSH VII Registry study. JACC Cardiovasc Interv 2017; 10: 986–95.
- Tonino PA, De Bruyne B, Pijls NH, et al. Fractional flow reserve versus angiography for guiding percutaneous coronary intervention. N Engl J Med 2009; 360: 213–24.
- Hong SJ, Kim BK, Shin DH, et al. Effect of Intravascular ultrasound-guided vs angiography-guided everolimus-eluting stent implantation: the IVUS-XPL randomized clinical trial. JAMA 2015; 314: 2155–63.
- Lee JM, Choi KH, Song YB, et al. Intravascular imaging-guided or angiography-guided complex PCI. N Engl J Med 2023; 388: 1668–79.
- Li X, Ge Z, Kan J, et al. Intravascular ultrasound-guided versus angiography-guided percutaneous coronary intervention in acute coronary syndromes (IVUS-ACS): a two-stage, multicentre, randomised trial. Lancet 2024; 403: 1855–65.
- Zimmermann FM, Ferrara A, Johnson NP, et al. Deferral vs. performance of percutaneous coronary intervention of functionally non-significant coronary stenosis: 15-year follow-up of the DEFER trial. Eur Heart J 2015; 36: 3182–88.
- Koo BK, Hu X, Kang J, et al. Fractional flow reserve or intravascular ultrasonography to guide PCI. N Engl J Med 2022; 387: 779–89.
- Zhang J, Gao X, Kan J, et al. Intravascular ultrasound versus angiography-guided drug-eluting stent implantation: the ULTIMATE trial. J Am Coll Cardiol 2018; 72: 3126–37.
- Witzenbichler B, Maehara A, Weisz G, et al. Relationship between intravascular ultrasound guidance and clinical outcomes after drug-eluting stents: the assessment of dual antiplatelet therapy with drug-eluting stents (ADAPT-DES) study. Circulation 2014;129: 463–70.




No comments yet!