Early intra-aortic balloon pump in heart failure complicated by cardiogenic shock: The Altshock-2 randomized clinical trial
Reported from ACC.25
Mirvat Alasnag provides her take on the Altshock-2 randomized clinical trial which was presented by Alice Sacco at ACC.25 in Chicago.
Background
Despite the development of standardized protocols and a wider experience with mechanical circulatory support (MCS) devices, mortality following cardiogenic shock (CS) continues to range between 30% and 50% depending on the stage and phenotype of CS.1-2 The use of intraaortic balloon counterpulsation (IABP), in particular, has declined compared with other devices following the IABP-SHOCK II trial that failed to show a significant reduction in 30-day mortality in patients with cardiogenic shock complicating acute myocardial infarction (AMI) for whom an early revascularization strategy was planned.3 In a retrospective analysis by Schrage and colleagues, the use of an Impella (microaxial pump) was not associated with lower 30-day mortality compared with matched patients from the IABP-SHOCK II trial treated with an IABP or medical therapy.4 The safety & feasibility of IABP as a bridge to durable Left Ventricular Assist Device or heart transplant without the need for escalation to more potent temporary mechanical circulatory support devices in ADHF patients has been demonstrated in a review by Brown et al.5 However, we still lack robust data.
It is important to note that there are differences between AMI-CS and acute decompensated heart failure (ADHF).2,6-7 ADHF-CS is often accompanied by a higher rate of end-organ and biventricular dysfunction, a longer hospital length of stay, increased use of heart replacement therapies (heart transplant or Left Ventricular Assist Device implantation) compared with AMI-CS.2,5 However, in-hospital mortality remains similar between the two.2,6 As such, it is imperative that targeted therapies are instituted for the different phenotypes of shock.
The aim of the ALTshock-2 Randomized Trial is to demonstrate the superiority of early IABP implantation over pharmacological therapies only in ADHF-CS, with respect to 60-day survival or successful bridge to heart replacement therapies.2
Methods
This is a prospective, randomized, multicenter, open-label study with blinded adjudicated evaluation of outcomes. Patients with ADHF-CS were randomized to early IABP implantation or vasoactive treatments. Inclusion criteria included all patients with hypotension or hypoperfusion (SCAI Class B-D), EF <35% and randomisation within 6 hours from the diagnosis of CS. The primary endpoint was 60 days survival or successful bridge-to-heart replacement therapy. The key secondary endpoints were 60-day overall survival; 60-day need for renal replacement therapy; in-hospital maximum inotropic score, maximum duration of inotropic/vasopressor therapy, and maximum sequential organ failure assessment score. The safety endpoints were in-hospital occurrence of bleeding events (BARC >3), vascular access complications and systemic (noncerebral) embolism.2 It is worth noting that the trial underwent a protocol amendment in response to the newly elaborated SCAI Classification. An analysis of 121 patients enrolled in the Altshock-2 registry after the initiation of the new inclusion & exclusion criteria, underscored the challenge of randomization in ADHF-CS. 47.9% of the 121 were likely suitable for enrollment after the protocol modification. Timely identification of patients was the limiting step.7
Results
The sample size calculation based on the anticipated mortality for ADHF-CS was 200 patients. After the first 100 patients the DSMB conducted an interim analysis of the first 100 patients and terminated the study early for a lack of benefit. As such, only a total of 53 patients were randomized to IABP and 48 to standard care. The median age was 60 years (885% men). SCAI stage B was noted in 28%, C 57% and D 15%. The use of inotropic agents and vasodilators was similar with epinephrine being the most commonly used agent (30%). Mechanical ventilation was more common in the standard of care group (44% vs 34%). A PA catheter was used in less than 50% of those enrolled. The primary endpoint was reached in 43 patients (81%) in the IABP group and 36 patients (75%) in the control group (P = 0.45). A total of 37 patients (37%) underwent HRT within the 60 days. Crossover of 6 patients (13%) were initially assigned to standard care to IABP. In terms of the secondary endpoints such as length of hospital stay and treatment escalation, they were similar between the groups. Bleeding and vascular complications were numerically higher in the IABP group.
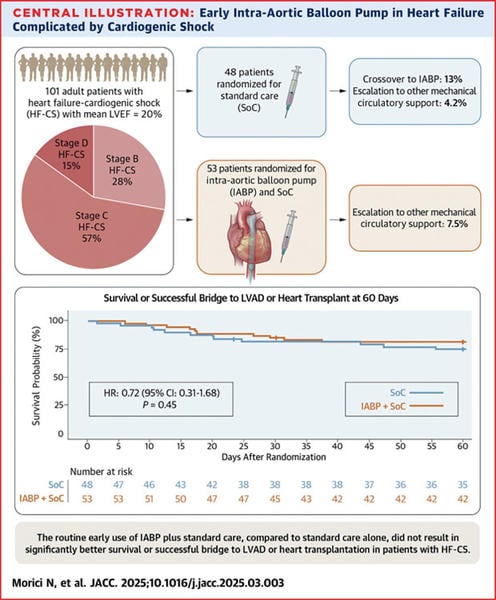
Figure: Adopted from Morici N, et al. JACC. 2025;10.1016/jjacc.2025.03.003
Interpretation
IABP failed to improve 60-day survival or bridge to HRT compared with standard care. There were numerous limitations of this study. Firstly, the sample size calculation was a gross underestimation even for the planned initial 200 patients. The low lactate level (mean 1.8mmol/L) could contaminate the results suggesting a poorly selected population further confirmed by the higher-than-usual survival rate in the standard-of-care population. The lack of standardization of IABP or vasopressor/inotropic support as well as the low use of PA catheters suggest inadequate optimization of MCS and shock management in the overall study population. That is further confirmed by the the consistent lack of benefit in all subgroup analyses including SCAI shock classification, lactate level, LVEF, and time of symptoms to arrival to CCU. The higher bleeding rates remain lower than with other MCS devices and suggest that other medical factors are contributors in such a population. Finally, a large number of patients were excluded due to a lack of informed consent. This warrants a careful assessment of the design of trials in critically ill patients.
References
- Chioncel O, Parissis J, Mebazaa A, Thiele H, Desch S, Bauersachs J, et al. Epidemiology, pathophysiology and contemporary management of cardiogenic shock—A position statement from the Heart Failure Association of the European Society of Cardiology. Eur J Heart Fail 2020; 22: 1315–1341. doi:10.1002/ejhf.1922
- Morici N, Marini C, Sacco A, Tavazzi G, Cipriani M, Oliva F, Rota M, De Ferrari GM, Campolo J, Frigerio G, Valente S, Leonardi S, Corrada E, Bottiroli M, Grosseto D, Cacciavillani L, Frigerio M, Pappalardo F; Altshock-2 Group. Early intra-aortic balloon pump in acute decompensated heart failure complicated by cardiogenic shock: Rationale and design of the randomized Altshock-2 trial. Am Heart J. 2021 Mar;233:39-47. doi: 10.1016/j.ahj.2020.11.017.
- Thiele H, Zeymer U, Neumann FJ, Ferenc M, Olbrich HG, Hausleiter J, Richardt G, Hennersdorf M, Empen K, Fuernau G, et al; IABP-SHOCK II Trial Investigators. Intraaortic balloon support for myocardial infarction with cardiogenic shock. N Engl J Med. 2012;367:1287–1296. doi: 10.1056/NEJMoa1208410.
- Schrage B, Ibrahim K, Loehn T, Werner N, Sinning JM, Pappalardo F, Pieri M, Skurk C, Lauten A, Landmesser U, et al. Impella support for acute myocardial infarction complicated by cardiogenic shock: A matched-pair IABP-SHOCK II trial 30-day mortality analysis. Circulation. 2019;139:1249–1258. doi: 10.1161/CIRCULATIONAHA.118.036614.
- Bertaina M, Morici N, Frea S, Garatti L, Briani M, Sorini C, Villanova L, Corrada E, Sacco A, Moltrasio M, Ravera A, Tedeschi M, Bertoldi L, Lettino M, Saia F, Corsini A, Camporotondo R, Colombo CNJ, Bertolin S, Rota M, Oliva F, Iannaccone M, Valente S, Pagnesi M, Metra M, Sionis A, Marini M, De Ferrari GM, Kapur NK, Pappalardo F, Tavazzi G. Differences between cardiogenic shock related to acute decompensated heart failure and acute myocardial infarction. ESC Heart Fail. 2023 Dec;10(6):3472-3482. doi: 10.1002/ehf2.14510.
- Brown MA, Sheikh FH, Ahmed S, Najjar SS, Molina EJ. Intra-Aortic Balloon Pump as a Bridge to Durable Left Ventricular Assist Device. J Am Heart Assoc. 2021 Aug 3;10(15):e019376. doi: 10.1161/JAHA.120.019376.
- M Milani, M Pagnesi, G Tavecchia, M Bertaina, S Frea, G M De Ferrari, A Sacco, G Tavazzi, C Colombo, A Pullara, L Villanova, C Sorini Dini, S Valente, N Morici, F Pappalardo, Altshock-2 investigators, Application of the Altshock-2 randomized trial enrollment criteria to the Altshock-2 registry population: are we missing someone?, European Heart Journal, Volume 44, Issue Supplement_2, November 2023. doi: 10.1093/eurheartj/ehad655.1130.




No comments yet!