Five-year outcomes of the randomised trial of fractional flow reserve guided PCI compared to coronary artery bypass grafting: FAME 3
Reported from ACC.25
Kalaivani Mahadevan provides her take on the five-year outcomes of the FAME 3 trial presented by William F. Fearon at ACC.25 in Chicago.
Why this study – the rationale/objective?
FAME 3 sought to provide up-to-date randomised data of contemporary PCI versus contemporary CABG in multivessel coronary disease1, utilising devices and techniques (2nd generation DES and FFR-guided PCI) not available or employed in earlier PCI vs CABG Trials such as SYNTAX, FREEDOM and BEST2-4.
How was it executed – the methodology?
The study initially reported at TCT in November 2021 with simultaneous publication in the NEJM. This was a multicentre, randomised trial seeking non-inferiority of FFR-guided PCI to CABG in multivessel CAD.
1,500 patients, deemed clinically suitable for both strategies at HEART Team MDT, with > 50 % angiographic stenosis in all three major epicardial coronaries, in the absence of left main disease, were randomised 1:1 to FFR-guided PCI or CABG.
Key exclusion criteria were cardiogenic shock, STEMI within 5 days and ejection fraction < 30 %.
Figure 1 summarises core patient, lesion and procedural demographics. The 1-year primary endpoint was major adverse cardiovascular and cerebrovascular events (MACCE), defined as death from any cause, myocardial infarction (MI), stroke (CVA), or repeat revascularization. The 5–year outcomes analysis utilised a primary composite endpoint of all–cause death, MI or CVA5.
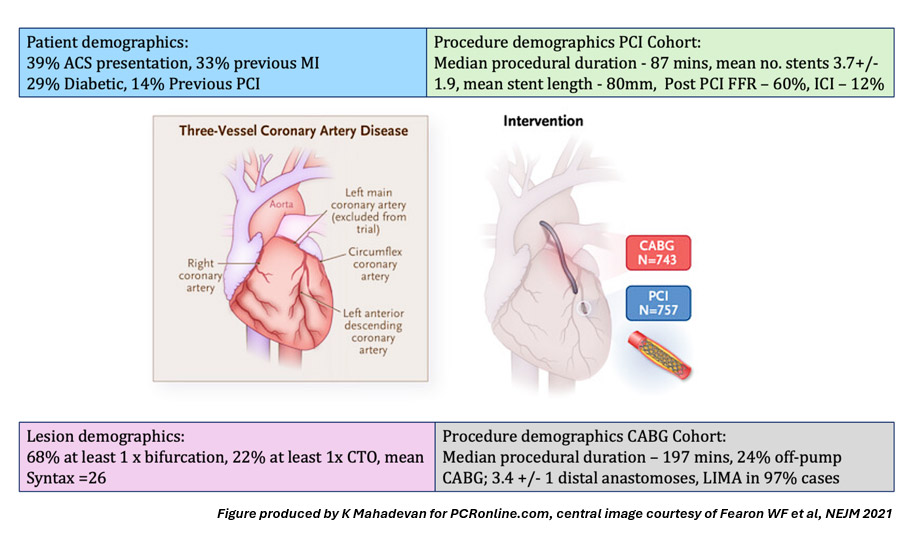
Figure 1: Patient, lesion and procedural demographics
What is the main result?
At one year, the primary endpoint occurred in 10.6 % and 6.9 % of patients in the FFR–guided PCI and CABG groups respectively, with HR 1.5 95 % CI 1.1 – 2.2] and P = 0.35 for non-inferiority (Figure 2). PCI, thereby failed to achieve non-inferiority to CABG1.
At 5–year analysis, the primary endpoint, a composite of all–cause death, MI and CVA occurred in 16 % and 14.1 % in FFR–guided PCI and CABG respectively, with HR of 1.16 [9 5% CI 0.89 – 1.52], P = 0.27, with no significant difference between the cohorts (Figure 2).
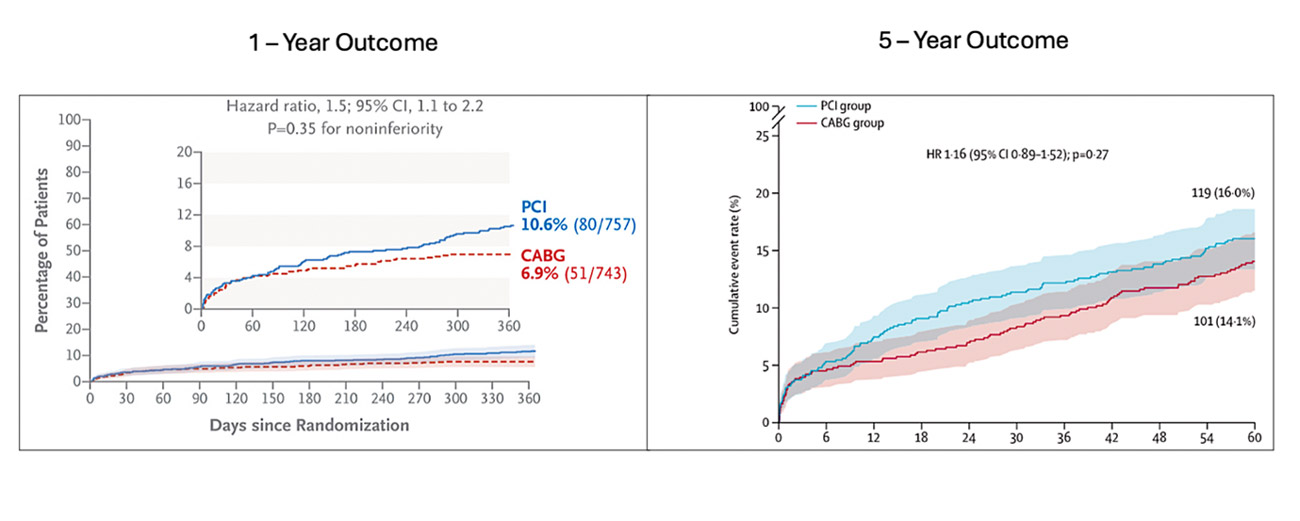
Figure 2: one- and five-year primary endpoint outcomes
Figure reproduced courtesy of Fearon WF et al, NEJM 2021 and The Lancet 2025.
Secondary endpoint analysis is summarised in Table 1. There was no difference in death but higher MI and repeat revascularisation rates in the PCI arm and higher CVA rate in the CABG arm5.
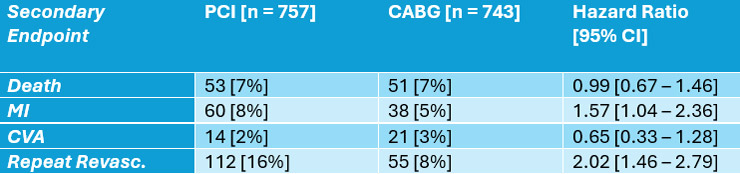
Table 1: 5 – Year Secondary Endpoint Outcomes
Table modified and reproduced courtesy of Fearon WF et al, Lancet 2025
Critical reading and the relevance for clinical practice
Discussion
The discussion for this review is tabulated below (Table 2), following an in-depth question-and-answer session with William Fearon, Chief Investigator and study lead. The discussion purpose was to deep dive into core questions, around trial methodology and results, that clinicians and readers may find to be interesting and helpful.
Table 2: Q+A interview session with William Fearon
Question | Answer/Discussion |
|---|---|
What was the rationale for changing the HR for the non-inferiority margin from 1.45 to 1.65 during recruitment? | Data from large PCI vs CABG trials (EXCEL, NOBLE, BEST) emerged with lower overall absolute event rates. It was important to take these data into account. |
Why was repeat revascularisation excluded from the 5–year endpoint (which consisted of death, MI and CVA)? |
|
Was a 5–year endpoint analysis performed with inclusion of repeat revascularisation? | Yes – with inclusion of repeat revascularisation into the composite endpoint, alongside death, MI and CVA, 5–year event rates were 18 % and 25 % in the CABG and PCI arms respectively, favouring CABG and is consistent with both the 1– and 3–year outcomes previously observed. |
What were the complete revascularisation rates in the PCI and CABG cohorts? | This data is currently in the process of being submitted for publication and will be available for review soon. |
Was CTCA to assess graft patency at 5 years considered in the original study design? | It was discussed and would have been good but could not be included due to limited funding and baseline trial costs. |
Was there a TVF/LTF difference in outcomes when post–PCI FFR was measured vs not measured? |
|
Do you think the low rate of intravascular imaging means that PCI outcomes could and should be better than reflected in this trial? | The low rate of ICI (12 %) mirrors imaging rates in the US and globally – hence trial outcome is generalisable to current real–world practice. The repeat revascularisation rate in the PCI cohort of FAME 3 (16 %) was comparable to that in Syntax II (13.8 %) which utilised a strategy of optimal PCI including high rates of IVUS. |
The late accrual benefit with CABG seen in Syntax was not seen in FAME 3 – why might this be? |
|
Limitations
The key study limitations included the following: the primary composite endpoint was powered only for 1-year outcomes but not for 5-year outcomes; follow-up was limited to 5 years; repeat revascularisation was excluded in the 5–year analysis; CABG–related clinically relevant endpoints (post-op AF, bleeding and re-hospitalisation) were not included; the low rate of complete arterial revascularisation in CABG arm and the low rate of intravascular imaging in the PCI arm, may underestimate the longer-term durability of both these strategies; finally, participation of a low number of female and ethnic minority patients which may reduce the external validity of the trial to these population groups.
Conclusion
This 5-year analysis of FAME 3, providing contemporary data on FFR-guided PCI versus CABG in non–left main, multivessel CAD, has both affirmed a reduction in outcome differences between CABG and PCI with the evolution of interventional techniques and provided further evidence to support individualised patient–centred shared decision-making around choice of revascularisation strategy.
The many decades long CABG – PCI relationship remains a crucial and symbiotic one for the optimal treatment of multivessel CAD.
Perhaps the next chapter in the history book of trials in this arena should seek to compare optimal PCI as seen in Syntax II6, with optimal CABG (maximising complete arterial grafting). Based on the data available to date, one would hypothesise yet further improvements in longer-term procedural durability and clinical outcomes for our patients with these strategies.
Acknowledgements:
With many thanks to Professor William Fearon for his time and participation in our Q+A interview session for this review.
References:
- Fearon WF, Zimmermann FM, De Bruyne B et al. Fractional Flow Reserve-Guided PCI as Compared with Coronary Bypass Surgery. N Engl J Med. 2022 Jan 13;386(2):128-137.
- Serruys PW, Morice MC, Kappetein AP et al. Percutaneous coronary intervention versus coronary-artery bypass grafting for severe coronary artery disease. N Engl J Med. 2009 Mar 5;360(10):961-72
- Farkouh ME, Domanski M, Sleeper LA et al. Strategies for multivessel revascularization in patients with diabetes. N Engl J Med. 2012 Dec 20;367(25):2375-84.
- Park SJ, Ahn JM, Kim YH et al. Trial of everolimus-eluting stents or bypass surgery for coronary disease. N Engl J Med. 2015 Mar 26;372(13):1204-12.
- Fearon WF, Zimmermann FM, Ding VY et al. Outcomes after fractional flow reserve-guided percutaneous coronary intervention versus coronary artery bypass grafting (FAME 3): 5-year follow-up of a multicentre, open-label, randomised trial. Lancet online 30th March 2025.
- Banning AP, Serruys P, De Maria GL et al. Five-year outcomes after state-of-the-art percutaneous coronary revascularization in patients with de novo three-vessel disease: final results of the SYNTAX II study. Eur Heart J. 2022 Mar 31;43(13):1307-1316.




No comments yet!