Effect of CYP2C19 genotype on ischemic outcomes during oral P2Y12 inhibitor therapy: a meta-analysis
Selected in JACC: Cardiovascular Interventions by S. Mcgrath , K. De Silva
Read more about this systematic review and meta-analysis which looked to ascertain if there is benefit in individualizing anti-platelet therapy on the basis of the CYP2C19 genotype.
References
Authors
Naveen L. Pereira, Charanjit Rihal, Ryan Lennon, Gil Marcus, Sanskriti Shrivastava, Malcolm R. Bell, Derek So, Nancy Geller, Shaun G. Goodman, Ahmed Hasan, Amir Lerman, Yves Rosenberg, Kent Bailey, M. Hassan Murad, and Michael E. Farkouh
Reference
J Am Coll Cardiol Intv. 2021 Apr, 14 (7) 739–750
Published
April 2021
Link
Read the abstractReviewers
Our Comment
Why this study? – the rationale/objective
Anti-platelet therapies are key part of a cardiologist’s armamentarium, with clopidogrel being a broadly prescribed drug that acts as an oral inhibitor of the platelet adenosine diphosphate P2Y12 receptor (P2Y12)1. Despite its widespread use, a proportion of patients (approximately 30 %) does not respond to clopidogrel due to a loss-of-function (LOF) alleles of the hepatic cytochrome p450 enzyme CYP2C19.
This LOF alleles stop clopidogrel being converted into its active metabolite, and can result in a higher incidence of ischaemic events2-4.
Recently, TAILOR-PCI, a large multi-centre randomised control trial (RCT) showed a signal (p = 0.06, 95 % CI of 0.43 to 1.02) of reducing ischaemic events (by 34 %) in CYP2C19 LOF carriers receiving ticagrelor compared with clopidogrel over 12 months, in patients presenting with an acute coronary syndrome (ACS)5.
However, this equivocal result, in addition to prior studies, having not shown a consistent clinical benefit and at present, there is insufficient evidence to confirm a clinically meaningful, causal effect of specific genotypes causing ischaemic events6,7.
This systematic review and meta-analysis looked to ascertain if there is benefit in individualizing anti-platelet therapy on the basis of the CYP2C19 genotype.
How was it executed? – the methodology
A detailed search strategy was developed and performed across several known databases (including OVID and Medline). Two independent investigators reviewed identified citation’s abstracts and titles to determine if they met the inclusion and exclusion criteria for the analysis. A second round of analysis of the remaining studies was carried out which consisted of a full text review. A third investigator adjudicated any discrepancies between reviewers. The Cochrane tool was used to assess the risk of bias in each RCT included, and the evidence was evaluated using a GRADE approach.
The target primary efficacy endpoint was defined as the composite CV death, myocardial infarction, stroke, stent thrombosis, and severe recurrent ischemia. The safety endpoint was defined as major or minor bleeding on the basis of thrombolysis in myocardial infarction (TIMI) criteria.
Adjusted effect sizes were extracted and pooled across studies using a random-effects model. The pooled effect was expressed as relative risk (RR) with 95 % CI. Analysis was conducted separately for CYP2C19 LOF carriers and non-carriers to explore the interaction between genotype and treatment effect, as well as a combined analysis that pooled both genotypes together to compare the two different drug regimes. Two analyses were performed, the first included solely RCTs, and the second included all studies. Furthermore, heterogeneity was assessed using I2, and an interaction test was conducted with two tailed p vales < 0.05 to indicate statistical significance.
What is the main result?
1,335 publications were identified by the initial literature search, of which 1,303 (97.6 %) did not meet the criteria for inclusion. The remaining 32 publications underwent full review, and a further 21 were excluded, as trial design did not allow assessment of outcomes based on LOF allele status. Data from TAILOR PCI was added as the initial search was conducted prior to trial publication. 12 studies were included in total, including 7 RCTs (15,949 patients) and 4 non-RCT studies (2,859 patients).
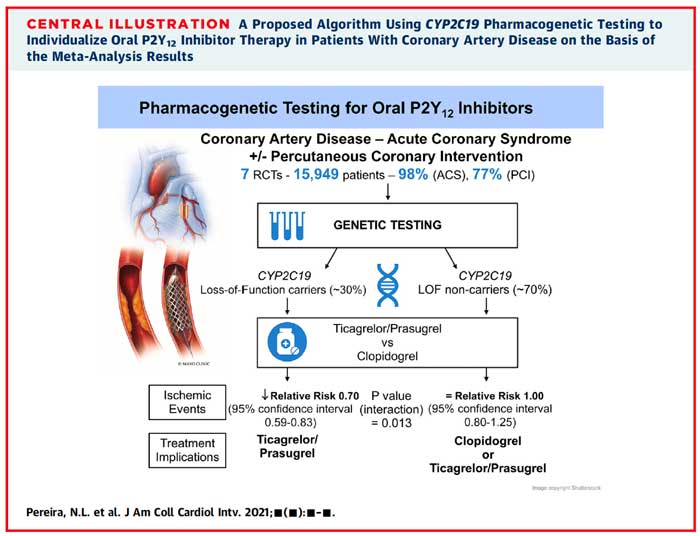
There were a total of 6,409 CYP2C19 LOF carriers in the initial analysis of RCTs. Results demonstrated a statistically significant reduction in ischaemic events occurred using ticagrelor or prasugrel opposed to clopidogrel in those carrying the LOF allele (RR 0.7, 95% CI 0.59 to 0.83). Analysis of 4 studies including 9,540 non-carriers did not show a significant risk reduction in the non-carrier group (RR 1, 95 % CI 0.8-1.25). The test of interaction for CYP2C19 genotype status was statistically significant (p = 0.013) suggesting that CYP2C19 genotype modifies outcomes. The secondary analysis was consistent with the results of the main analysis, an additional 4 observational studies were found, and adding them provided the same conclusions (p value of the test of interaction < 0.001). Furthermore, there was no statistically significant difference in the risk for major or minor bleeding in the LOF carrier group with the use of ticagrelor or prasugrel compared to clopidogrel based on TIMI criteria (RR 0.91, 95% CI 0.64 to 1.30). The test for interaction between metabolizer type and treatment effect was non-significant (p= 0.67) and was re-affirmed in the secondary analysis (p = 0.92). The heterogeneity of treatment effect was minimal: I2 < 50 % in all analyses stratified by CYP2C19 genotype.
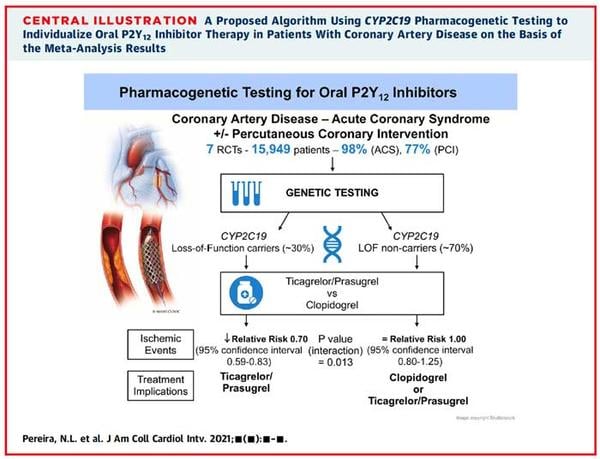
Source: JACC Cardiovascular Interventions
Critical reading:
In this review, those carrying the CYP2C19 LOF allele with ACS were observed to have improved ischaemic outcomes with prasugrel or ticagrelor when compared to clopidogrel, with no statistically significant difference in ischaemic outcomes in those identified as non-carriers. The test of interaction for CYP2C19 genotype status was statistically significant (p = 0.013), suggesting that CYP2C19 genotype modifies outcomes. Furthermore, there was no significant difference in the risk of bleeding in either group based on TIMI criteria (p-value of interaction 0.67).
The study design was robust, with appropriate inclusion and exclusion criteria, detailed search methodology, and appropriate random-effects model given the heterogeneity. However, one major issue with the current analysis was that a large proportion of patients (97.6 %) identified in the initial search were excluded. Importantly, 45 % (529) of the original papers were not original studies and composed of letters, case reports, and reviews, and therefore did not meet inclusion criteria. 186 studies had missing genotype information, and a further 101 did not have an adequate two-arm design. There were 109 studies excluded as they did not treat ACS patients, and the remaining studies did not meet the criteria for various other reasons. The second round of analysis excluded a further 21 studies as it was not possible to assess LOF allele carrier versus non-carrier status, which again further reduced the patient population being studied. Additionally, a potential source of bias was that studies were only included if English was the primary language, which may have led to important data being excluded.
The relevance for clinical practice:
The authors suggest that genotyping LOF carriers versus non-carriers could be beneficial in the selection of antiplatelet therapy on an individual basis given that 30 % of the population carry one of the LOF alleles1. The benefit in ischaemic outcomes with genotype-guided therapy would potentially apply if we adopted a clopidogrel-for-all strategy to antiplatelet therapy in ACS. However, current recommendations by the ESC for antiplatelet therapy in ACS is that patients should be treated with potent P2Y12 inhibitor therapy such as ticagrelor or prasugrel, maintained over twelve months, unless contraindicated or high risk of excessive bleeding8,9. Therefore, we question the potential benefit that would be obtained by genotype-guided therapy undertaken on a systematic basis in contemporary practice, in the context that clopidogrel is no longer the first-line treatment in the ACS setting.
Another observation stated by the authors in favour of genotype-guided therapy is that it may reduce the risk of bleeding with targeted therapy. However, the current analysis did not show any significant difference in bleeding rates amongst the groups (RR 0.91, 95 % CI 0.64 to 1.30 p-interaction p = 0.67). Other studies have, using the BARC classification rather than TIMI criteria for bleeding, shown lower minor bleeding rates with the use of clopidogrel5,10, which the authors propose could be significant if 70 % (non-carriers) receive clopidogrel over prasugrel or ticagrelor. Critically, however, there was no significant difference in rates of major bleeding, (HR 1.05 (0.45 to 2.44) p-value 0.9 in5). Therefore on balance, genotype-guided switching of antiplatelet therapies, whilst may potentially reduce minor bleeding is unlikely to have a significant impact on overall clinical outcomes. Finally, prior to consideration of more widespread utilization of genotype testing (even for with the small numbers of patients this is likely to have an impactful difference upon), practical questions regarding implementation need to be considered, such as the ease and availability of testing for the LOF allele and the overall cost-effectiveness of this process.
Whilst this is a very interesting analysis and reaffirms the importance of considering genotype-guided therapies and personalization of care in coronary disease moving forward, there continues to be limited evidence to support the use of genotype-guided antiplatelet prescribing on a systematic basis based upon the data outlined in this important work.
References:
- Pereira, N.L., Rihal, C.S., So, D.Y., Rosenberg, Y., Lennon, R.J., Mathew, V., Goodman, S.G., Weinshilboum, R.M., Wang, L., Baudhuin, L.M. and Lerman, A., 2019. Clopidogrel Pharmacogenetics: State-of-the-Art Review and the TAILOR-PCI Study. Circulation: Cardiovascular Interventions, 12(4), p.e007811.
- Mega, J.L., Simon, T., Collet, J.P., Anderson, J.L., Antman, E.M., Bliden, K., Cannon, C.P., Danchin, N., Giusti, B., Gurbel, P. and Horne, B.D., 2010. Reduced-function CYP2C19 genotype and risk of adverse clinical outcomes among patients treated with clopidogrel predominantly for PCI: a meta-analysis. Jama, 304(16), pp.1821-1830.
- Holmes, D.R., Dehmer, G.J., Kaul, S., Leifer, D., O’Gara, P.T. and Stein, C.M., 2010. ACCF/AHA Clopidogrel Clinical Alert: Approaches to the FDA “Boxed Warning” A Report of the American College of Cardiology Foundation Task Force on Clinical Expert Consensus Documents and the American Heart Association Endorsed by the Society for Cardiovascular Angiography and Interventions and the Society of Thoracic Surgeons. Journal of the American College of Cardiology, 56(4), pp.321-341.
- Pereira, N.L. and Weinshilboum, R.M., 2009. Cardiovascular pharmacogenomics and individualized drug therapy. Nature Reviews Cardiology, 6(10), pp.632-638.
- Pereira, N.L., Farkouh, M.E., So, D., Lennon, R., Geller, N., Mathew, V., Bell, M., Bae, J.H., Jeong, M.H., Chavez, I. and Gordon, P., 2020. Effect of genotype-guided oral P2Y12 inhibitor selection vs conventional clopidogrel therapy on ischemic outcomes after percutaneous coronary intervention: the TAILOR-PCI randomized clinical trial. Jama, 324(8), pp.761-771.
- Ogawa, H., Isshiki, T., Kimura, T., Yokoi, H., Nanto, S., Takayama, M., Kitagawa, K., Nishikawa, M., Miyazaki, S., Ikeda, Y. and Nakamura, M., 2016. Effects of CYP2C19 allelic variants on inhibition of platelet aggregation and major adverse cardiovascular events in Japanese patients with acute coronary syndrome: The PRASFIT-ACS study. Journal of cardiology, 68(1), pp.29-36.
- Dong, P., Yang, X. and Bian, S., 2016. Genetic polymorphism of CYP2C19 and inhibitory effects of ticagrelor and clopidogrel towards post-percutaneous coronary intervention (PCI) platelet aggregation in patients with acute coronary syndromes. Medical science monitor: international medical journal of experimental and clinical research, 22, p.4929.
- Collet, J.P., Thiele, H., Barbato, E., Barthélémy, O., Bauersachs, J., Bhatt, D.L., Dendale, P., Dorobantu, M., Edvardsen, T., Folliguet, T. and Gale, C.P., 2021. 2020 ESC Guidelines for the management of acute coronary syndromes in patients presenting without persistent ST-segment elevation: the Task Force for the management of acute coronary syndromes in patients presenting without persistent ST-segment elevation of the European Society of Cardiology (ESC). European heart journal, 42(14), pp.1289-1367.
- Ibanez, B., James, S., Agewall, S., Antunes, M.J., Bucciarelli-Ducci, C., Bueno, H., Caforio, A.L.P., Crea, F., Goudevenos, J.A., Halvorsen, S., Hindricks, G., Kastrati, A., Lenzen, M.J., Prescott, E., Roffi, M., Valgimigli, M., Varenhorst, C., Vranckx, P., Widimský P., 2018. 2017 ESC Guidelines for the management of acute myocardial infarction in patients presenting with ST-segment elevation: The Task Force for the management of acute myocardial infarction in patients presenting with ST-segment elevation of the European Society of Cardiology (ESC). European heart journal. 39(2):119-177
- Claassens, D.M., Vos, G.J., Bergmeijer, T.O., Hermanides, R.S., Van’t Hof, A.W., Van Der Harst, P., Barbato, E., Morisco, C., Tjon Joe Gin, R.M., Asselbergs, F.W. and Mosterd, A., 2019. A genotype-guided strategy for oral P2Y12 inhibitors in primary PCI. New England Journal of Medicine, 381(17), pp.1621-1631.




No comments yet!