07 Jun 2021
Top Interventional Trials presented at ACC.21
Reported from the American College of Cardiology Scientific Sessions 2021
Ali Nazmi Calik provides a summary and key take-aways and infographics of 9 major trials of interest to interventional cardiologists presented at ACC.21
A. Antithrombotic / anticoagulant therapy
1. ADAPTABLE: Aspirin Dosing: A Patient-Centric Trial Assessing Benefits and Long-term Effectiveness
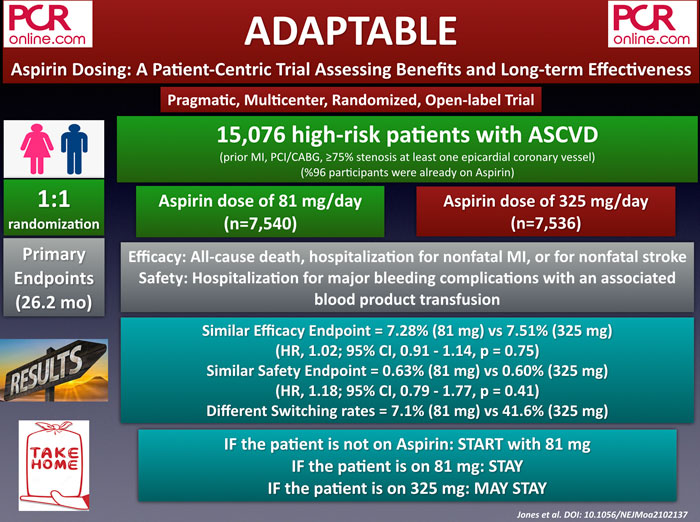
ADAPTABLE is a pragmatic, multicenter, randomized, and open-label study that sought to evaluate low (81 mg) and high doses of aspirin (325 mg) in patients with established atherosclerotic cardiovascular disease (ASCVD). The study enrolled 15,076 patients, of whom 96 % were already on aspirin. The patients were randomly assigned to 81 mg (n = 7,540) and 325 mg (n = 7,536) of aspirin and followed for a median duration of 26.2 months.
The primary efficacy outcome of the study was defined as all-cause death, MI, or stroke at 1 year and occurred in 7.3 % of the aspirin 81 mg group compared with 7.5 % of the aspirin 325 mg group (p = 0.75). Similarly, the primary safety outcome, which is major bleeding requiring blood transfusion at 1 year, was identical between the groups (0.63 % vs 0.60 %, p = 0.41). Nonetheless, patients assigned to 325 mg had a higher incidence of dose switching than those assigned to 81 mg (41.6 % vs. 7.1 %) and fewer median days of exposure to the assigned dose.
In the ADAPTABLE trial, involving patients with established ASCVD, no significant differences were found between taking low or high dose aspirin in reducing cardiovascular events or major bleeding. To conclude, while it is reasonable to initiate 81 mg aspirin to aspirin naive patients, patients who are already on 81 mg or 325 mg aspirin may continue to take the same doses.
Take home message:
- if the patient is not on Aspirin, START w/ 81mg
- if the patient is on 81 mg Aspirin, STAY
- if the patient is on 325mg, MAY STAY
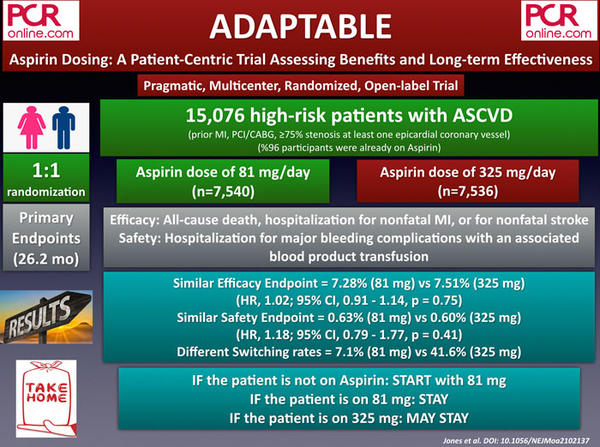
2. TALOS-AMI: TicAgrelor Versus CLOpidogrel in Stabilized Patients With Acute Myocardial Infarction
TALOS-AMI is a prospective, multicenter, randomized, and open-label study aiming to evaluate the efficacy and safety of de-escalation to clopidogrel-based DAPT strategy in stabilized acute myocardial infarction (AMI) patients. The study enrolled a total of 2,697 AMI patients who underwent PCI with DES, and received 1 month ASA + ticagrelor without any adverse clinical event. One month after index PCI, the participants were randomly assigned to ASA + clopidogrel (de-escalation, n = 1,349) and ASA + ticagrelor (n = 1,348) groups.
The composite primary endpoint defined as cardiovascular death, MI, stroke, Bleeding Academic Research Consortium (BARC) bleeding 2,3, or 5 was observed 4.6 % of ASA + clopidogrel group and 8.2 % of ASA + ticagrelor group (p for noninferiority < 0.001, p for superiority < 0.001).
Whereas the secondary outcome of CV death, MI, stroke was similar between the groups (2.1 % vs 3.1 %, p = 0.15), BARC 2,3, or 5 bleeding was significantly lower in the de-escalation group (3% vs 5.6%, p = 0.001).
In AMI patients who are event-free under ASA + ticagrelor DAPT during the first 30 days of index PCI, de-escalation to clopidogrel-based DAPT was superior to continuing ticagrelor-based DAPT in terms of net clinical benefit, which is primarily driven by lowering bleeding events.
Take home message:
In AMI patients event-free during first 30 days of index PCI, de-escalation to clopidogrel #DAPT was superior to ticagrelor #DAPT.
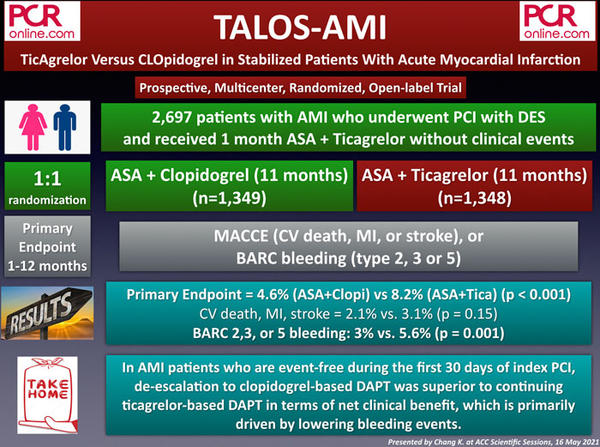
3. HOST-EXAM: Aspirin vs Clopidogrel During Chronic Maintenance Monotherapy After Percutaneous Coronary Intervention
The HOST-EXAM is a prospective, multicenter, randomized, and open-label study that included 5,438 enrollees who received DAPT for an event-free 6-18 months after PCI with DES. At the time of PCI, 25.5 % had stable angina, 35.5 % had unstable angina, 19.4 % had non-ST segment elevation MI, and 17.2 % had STEMI. Participants were randomized to either clopidogrel (n = 2,710) or aspirin (n = 2,728) monotherapy with a 1:1 fashion.
The primary outcome was a composite of all-cause death, non-fatal myocardial infarction, stroke, readmission due to acute coronary syndrome, and Bleeding Academic Research Consortium (BARC) bleeding type 3 or greater at 2 years.
The primary outcome occurred in 152 (5.7 %) patients in the clopidogrel group and 207 (7.7 %) in the aspirin group (p = 0.0035). Secondary outcomes including thrombotic composite outcome (3.7 % vs. 5.5 %, p = 0.003) and any bleeding (2.3 % vs. 3.3 %, p = 0.003) were also in favour of clopidogre monotherapy.
Despite positive results favouring clopidogrel over aspirin, the East Asian cohort of the study, which is known to have relatively low thrombotic risk, raises the concern of whether these findings can be reproduced in different regions of the globe. Also, the follow-up time (24 months) may be too short for providing a brief conclusion. Without a doubt, the already launched ‘HOST-EXAM extended study’ with a 10-years of median follow-up will provide more concrete data on this issue.
Take home message:
In patients requiring indefinite monotherapy after PCI, clopidogrel was superior to aspirin in preventing future adverse events.
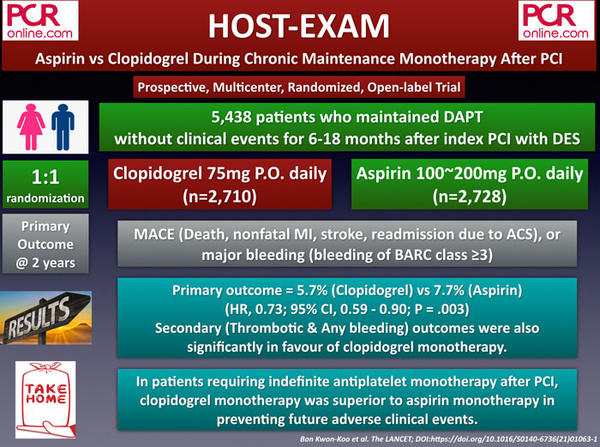
4. ATLANTIS: Apixaban to Lower All Cardiovascular and Neurologic Events After TAVI
The ATLANTIS is a prospective, multicenter, randomized, and open-label trial that was conducted to evaluate the efficacy and safety of apixaban 5 mg BID compared with standard of care (antiplatelet therapy [APT] or oral anticoagulant [OAC]) in post-TAVI patients.
A total of 1,500 patients undergoing successful TAVI procedure were stratified according to having an indication for OAC or not, and randomly assigned to either apixaban 5 mg BID vs vitamin K antagonist (VKA) (21 %) [stratum 1 = among patients with an indication for OAC, n = 451], or apixaban 5 mg BID vs single APT (15 %) / dual APT (57 %) [stratum 2 = among patients without an indication for OAC, n = 1,049].
The primary endpoint, consisting of death, stroke, myocardial infarction (MI), systemic emboli, intracardiac or valve thrombosis, deep vein thrombosis/pulmonary embolism, or major bleeding at 1 year, was observed in 18.4 % of the apixaban group, and 20.1 % of the standard of care group (p = NS). The results also did not significantly differ between stratum 1 (apixaban vs VKA: 21.9 % vs 21.9 %) and stratum 2 (apixaban vs APT: 16.9 % vs 19.3 %).
Excluding bioprosthetic valve thrombosis, which was significantly higher in the standard of care group (1.1 % vs 4.7 %, p < 0.05, mainly driven by the patients in the APT group), the composite endpoint was found to be occurring higher in the apixaban group (17.8 % vs 16.1 %, p = significant). Regarding the primary safety endpoint, including life-threatening, disabling, or major bleeding, there were no significant differences between the groups.
The ATLANTIS trial did not show any benefit of apixaban over the standard of care (VKA if an indication for OAC; APT if no indication for OAC) in post-TAVI patients, except lowering valve thrombosis that also did not provide any clinical benefit.
Take home message:
In post-#TAVR patients, Apixaban is not superior to standard of care despite a reduction of valve leaflet thrombosis.
B. Structural interventions
1. LAAOS III: Left Atrial Appendage Occlusion Study III
The LAAOS III is a prospective, multicenter, randomized, and open-label study that sought to assess the efficacy and safety of surgical left atrial appendage (LAA) closure in atrial fibrillation/flutter patients undergoing on-pump heart surgery for other reasons.
A total of 4,770 AF/flutter patients with a CHA2DS2-VASc score of at least 2 (mean CHA2DS2-VASc score was 4.2) were enrolled and randomized to either surgical closure of LAA or no closure. Oral anticoagulation use at 3 years was noted in 75 % of the LAA closure group and 78 % of the no closure group.
The primary efficacy endpoint, defined as ischemic stroke or systemic embolism, occurred in 4.8 % of the LAA closure group and 7.0 % of the no closure group (p = 0.001) at a median of 3.8 years. Perioperative bleeding, heart failure, or death did not differ significantly between the study groups.
Among patients with atrial fibrillation who had undergone cardiac surgery, the concomitant LAA closure reduced the risk of ischemic stroke or systemic embolism compared to no closure.
The results of the LAAOS III study should not be misinterpreted as LAA closure is an alternative to eliminate OAC therapy. Although a subgroup analysis indicated that patients who were not on oral anticoagulation at baseline still appeared to benefit from surgical occlusion of the LAA, the greatest benefit was additive to OAC therapy that was continued by a vast majority of participants. Also, the LAAOS III study's encouraging results may trigger the question of whether transcatheter LAA closure on top of long-term OAC will provide benefit over long-term OAC only.
Take home message:
Among patients with AF undergoing cardiac surgery, surgical LAA occlusion reduces ischemic stroke by 33%, compared to no occlusion.
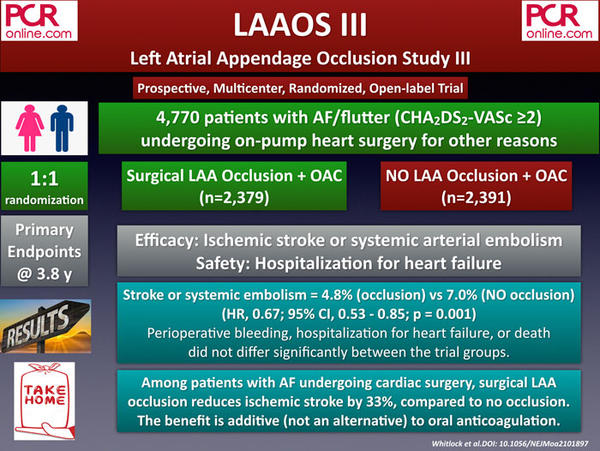
2. A Report From the NCDR LAAO Registry: One-year Clinical Outcomes Following Watchman Transcatheter Left Atrial Appendage Occlusion For Stroke Prevention In Patients With Atrial Fibrillation
The analysis of ACC’s National Cardiovascular Data Registry (NCDR) LAAO Registry included 36,681 patients who received a Watchman device between 2016 and 2018 and were eligible for 1-year follow-up. The primary endpoint of the study was the rate of ischemic stroke. Secondary endpoints included death, systemic embolism, and major bleeding.
The mean age of the study population was 76 years, and 70 % of those were at high risk for major bleeding, a clinical feature making them not eligible for receiving long-term NOAC. The mean CHA2DS2-VASc and HAS-BLED scores of the participants were 4.8 and 3.0, respectively.
After one year of follow-up, the ischemic stroke was observed significantly (77 %) lower than the expected rates in the study group (1.53 % vs 6.64 %), based on the patients’ CHA2DS2-VASc scores. Speaking for the secondary endpoints, 8.52 % of the patients died, 6.2 % of the patients experienced a major bleeding event, and only 0.7 % of the patients suffered from a systemic embolism at one year.
In conclusion, NCDR LAAO registry supports the early-to-mid term efficacy and safety of LAA occlusion with the WATCHMAN device, particularly in patients with contraindications to OAC, who were not included in the previous PROTECT-AF and PREVAIL trials.
Take home message:
NCDR LAAO registry supports early to mid-term efficacy & safety of Watchman LAAO in patients with contraindications to OAC.
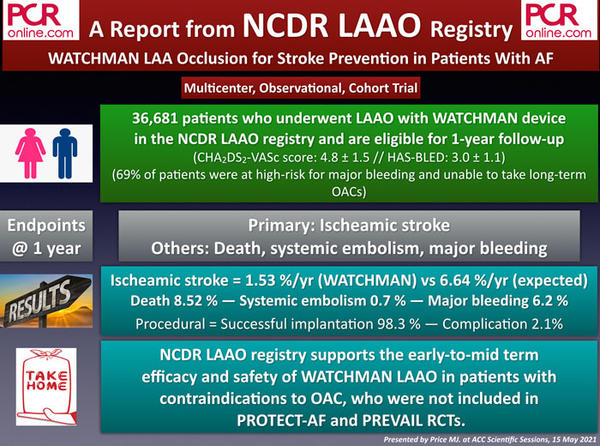
3. TRISCEND: Transfemoral Tricuspid Valve Replacement In Patients With Tricuspid Regurgitation
The TRISCEND is a single-arm, multicenter and prospective trial including patients with severe tricuspid regurgitation (TR) undergoing transfemoral tricuspid valvular replacement (TVR) by Edwards EVOQUE system.
Based on the etiology, 68 % of the patients had functional, 11 % had degenerative, and the rest had mixed or other etiologies. The vast majority of the patients (92 %) had at least severe TR (severe in 46 %, massive in 29 %, and torrential in 15 %).
The early results at 30 days showed a significant reduction in TR severity (98 % mild or less) and significant improvement in NYHA class, 6-min walking distance, and Kansas City Cardiomyopathy Questionnaire (KCCQ) score.
Early favourable results of the TRISCEND trial demonstrating the technical feasibility and safety of EVOQUE transfemoral TVR system led to the initiation of the TRISCEND II pivotal randomized trial, which will compare EVOQUE plus optimal medical therapy versus optimal medical therapy alone.
Take home message:
In patients with clinically significant TR, EVOQUE transfemoral system provided significant TR reduction, and symptomatic improvement at 30 days.
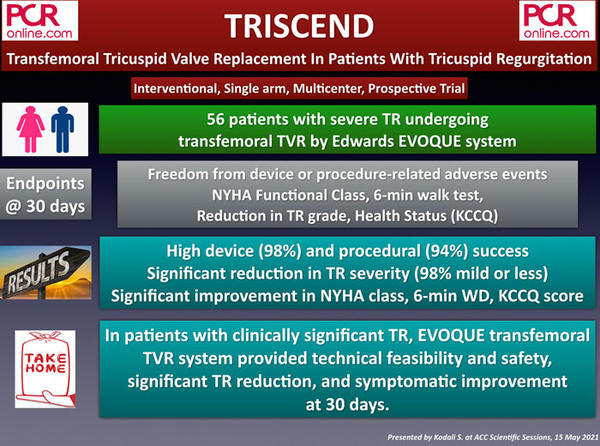
C. Coronary physiology assessment
1. FLOWER-MI: FFR-guided vs Angio-guided Multivessel Revascularization In ST-Elevation Myocardial Infarction Patients
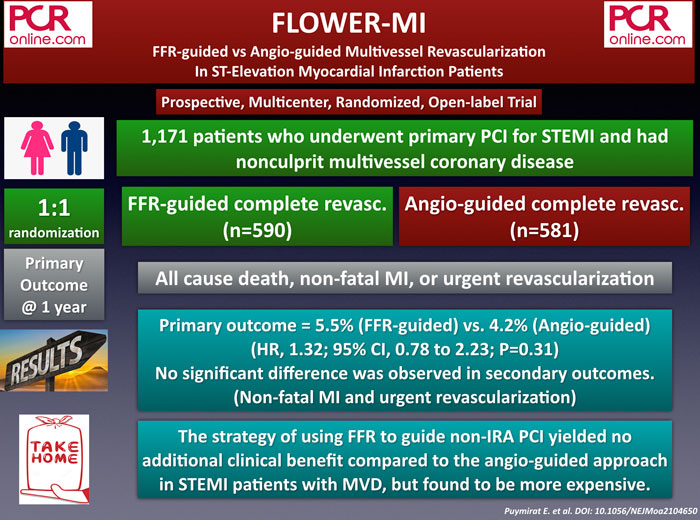
The FLOWER-MI is a prospective, multicenter, randomized, and open-label study that compared FFR guided complete revascularization (CR) with angiography-guided CR in STEMI patients undergoing primary PCI for their culprit lesions. A total of 1,171 patients were enrolled and randomly assigned to FFR-guided (n=590) and angio-guided (n = 581) CR group. Almost all patients (97 %) had staged procedures for non-culprit lesions.
The primary endpoint, including all-cause death, non-fatal MI, or urgent revascularization at 12 months, occurred in 5.5 % of the FFR-guided group and 4.2 % of the angio-guided group (p = 0.31). In terms of individual components, death (1.5 % vs 1.7 %), non-fatal MI (3.1 % vs 1.7 %), unplanned hospitalization for urgent revascularization (2.6 % vs 1.9 %) were not different between the groups. Of note, the median cost of FFR-guided strategy was more expensive than angiography-guided strategy (€ 8,832 vs € 8,322; P < 0.01).
In short, the strategy of using FFR to guide non-IRA PCI for complete revascularization provided no additional clinical benefit compared to the angio-guided approach in STEMI patients with MVD but found to be more expensive.
Take home message:
Using FFR to guide non-IRA PCI yielded no additional benefit compared to angio in #STEMI patients with MVD.
Read the related EAPCI/PCR Journal Club review by Nicola Ryan and Michele Pighi here.
D. Anti-hypertensive interventions
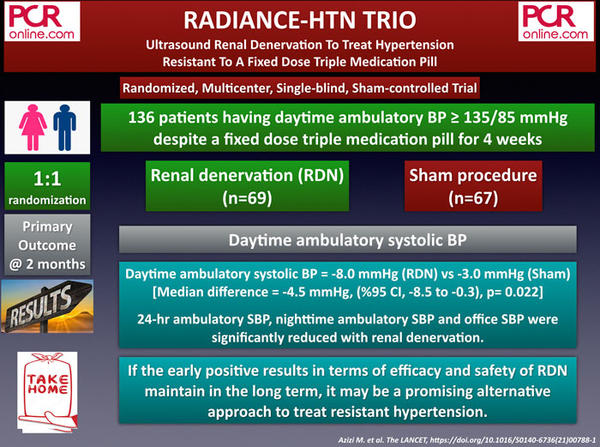
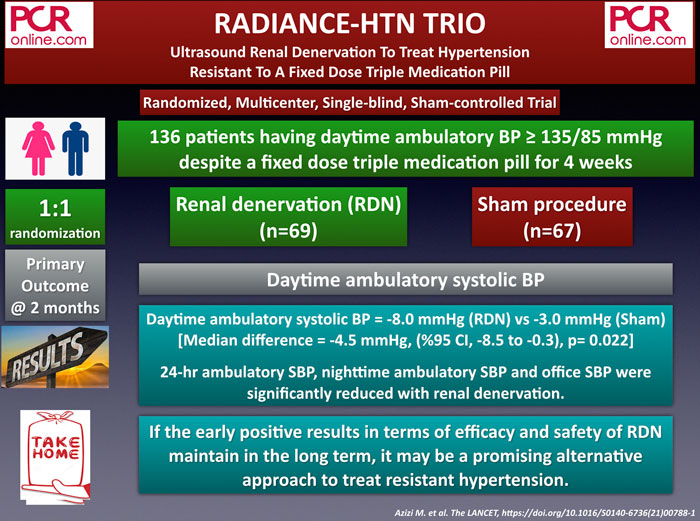
1. RADIANCE-HTN TRIO: Ultrasound Renal Denervation To Treat Hypertension Resistant To A Fixed Dose Triple Medication Pill
The RADIANCE-HTN TRIO is a single-blind, randomized, multicenter and sham-controlled trial evaluating the efficacy and safety of renal denervation (RDN) in 136 patients having daytime ambulatory BP ≥ 135/85 mmHg despite a fixed-dose triple medication pill (calcium channel blocker, angiotensin receptor blocker, and a thiazide diuretic) for four weeks.
The primary outcome, daytime ambulatory systolic blood pressure at two months, significantly reduced with RDN (- 8.0 mmHg) compared to sham-procedure (-3.0 mmHg) (mean difference: - 4.5 mmHg, p=0.022). In addition, significant between-group reductions in 24-hour ambulatory systolic blood pressure, nighttime ambulatory systolic blood pressure, and office-based systolic blood pressure were observed at two months. There were no differences in safety outcomes between the two groups.
The early results of the RADIANCE-HTN TRIO trial, which are in line with the previous RADIANCE-HTN SOLO study, offer RDN as an additional tool for treating hypertension, particularly if the promising results are durable in the longterm.
Take home message:
If early results are maintained in the LT, RDN may be a promising alternative to treat resistant #hypertension.